Sustained release composition and preparation method thereof
A technology of sustained release and composition, applied in the field of medicine, can solve problems such as insufficient dissolution, achieve the effects of reducing peak concentration, increasing drug loading, and increasing strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~18
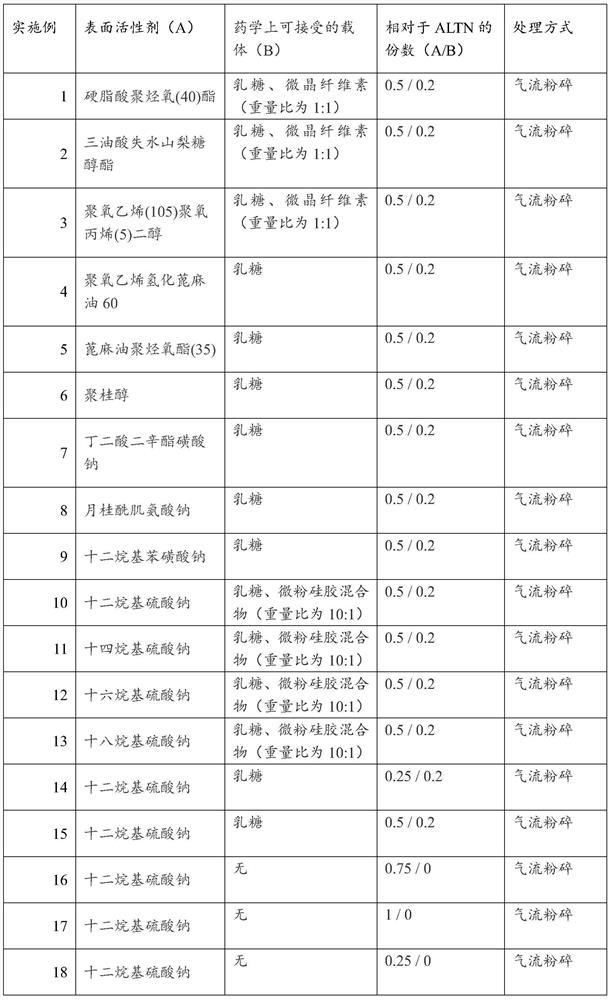
[0054] For Examples 1 to 18, ALTN hydrochloride, surfactants, and pharmaceutically acceptable carriers are mixed according to a certain ratio and then physically mixed to obtain various combinations of ALTN and surfactants shown in Table 1. mixture. Wherein the weight of ALTN hydrochloride is calculated as ALTN, and the weight of ALTN is used as a benchmark to count as 1 part.
[0055] Table 1
[0056]
Embodiment 19~22
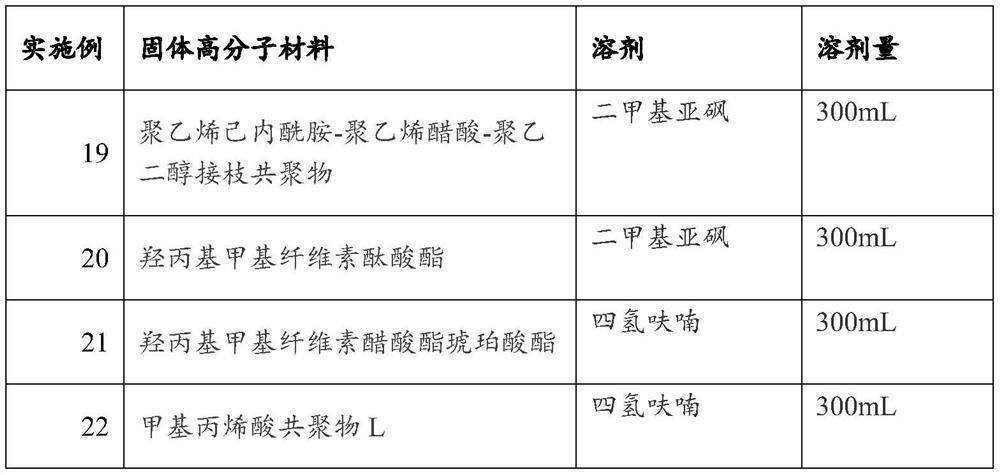
[0058] For Examples 19-22, ALTN hydrochloride (2.5 g as ALTN) and the solid polymer material (5.60 g) shown in Table 2 were put in a container, the corresponding solvent was added, and the mixture was stirred until dissolved. The obtained solution is spray-dried at about 100° C., followed by drying under reduced pressure to obtain a solid dispersion of ALTN.
[0059] Table 2
[0060]
Embodiment 23~26
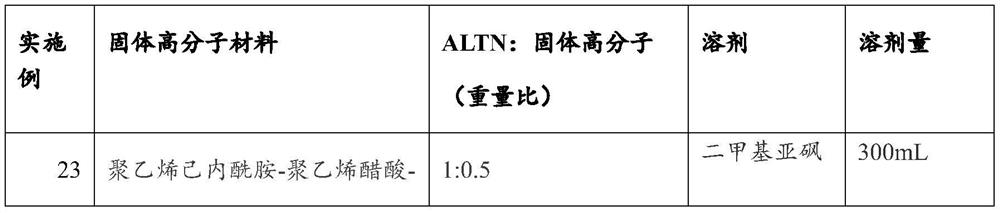
[0062] For Examples 23-26, different amounts of ALTN hydrochloride and solid polymer materials shown in Table 3 were placed in containers, corresponding solvents were added, and the mixture was stirred until dissolved. The obtained solution is spray-dried at about 100° C., followed by drying under reduced pressure to obtain the ALTN solid dispersion.
[0063] table 3
[0064]
[0065]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com