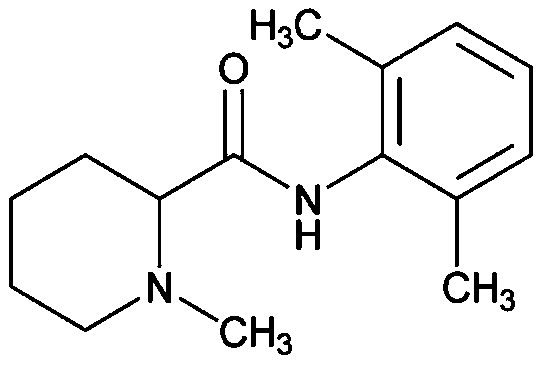
Mepivacaine xinafoate and long-acting sustained release preparation thereof
A technology of mepivacaine xinafoate and mepivacaine, which is applied to medical preparations containing active ingredients, anesthetics, anti-inflammatory agents, etc., can solve the problems of short duration of anesthesia, short biological half-life, and high medical and nursing costs , to achieve the effect of prolonging anesthesia time, prolonging half-life and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 mepivacaine xinafoate
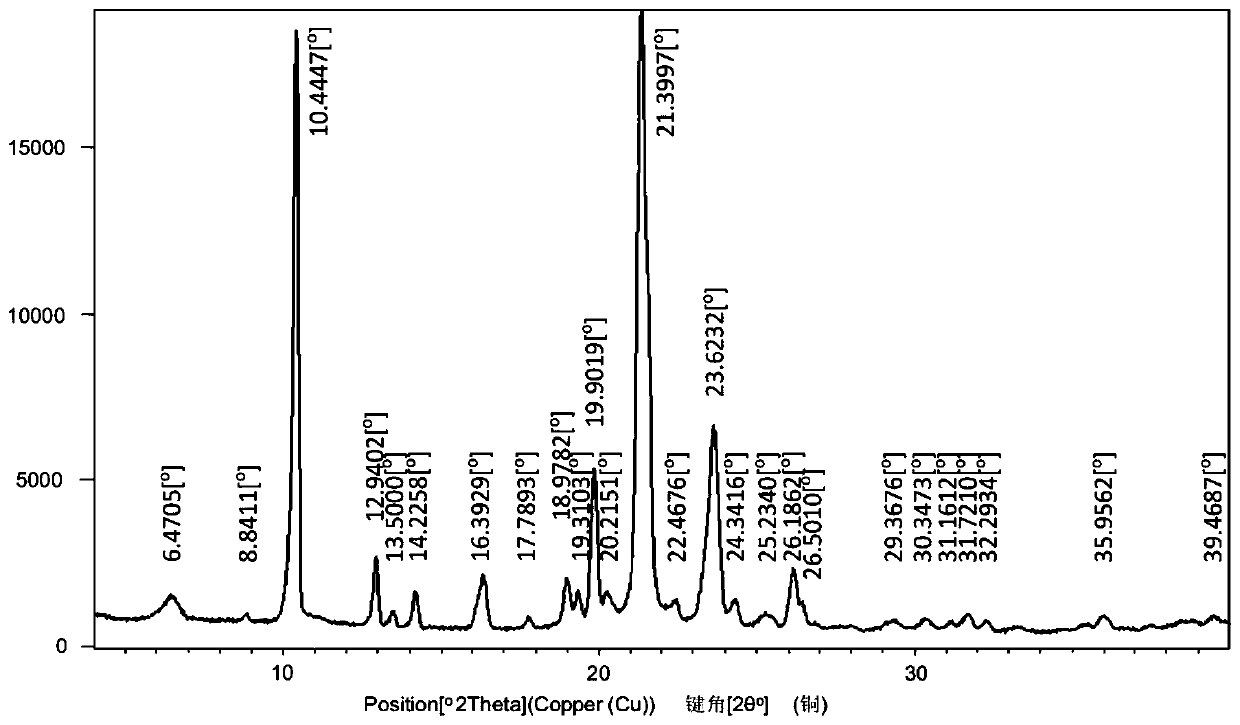
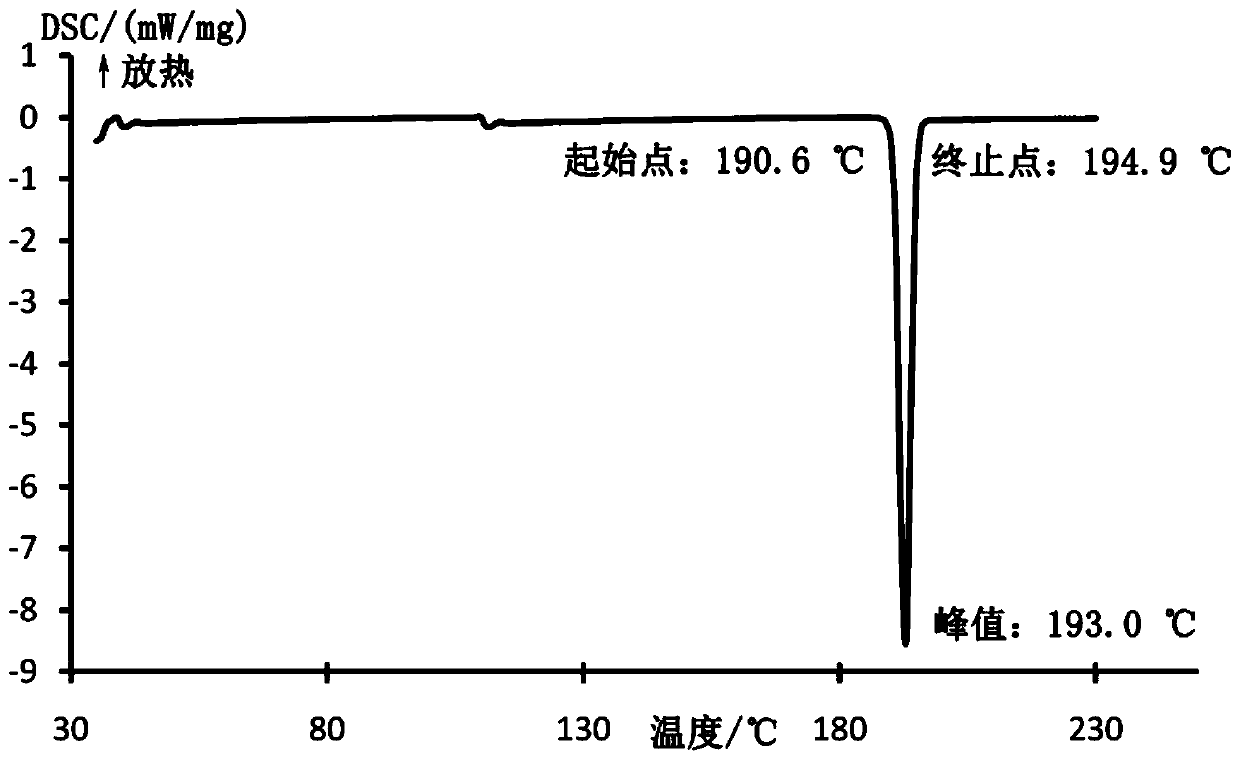
[0032] Mepivacaine base (12.3 g; 50 mmol) and xinafoic acid (9.41 g; 50 mmol) were added to absolute ethanol (300 mL), and stirred at 60° C. for 5 hours. Subsequently, 2L of water was added to the reaction solution, and a white solid was precipitated, which was filtered and vacuum-dried to obtain mepivacaine xinafoate salt. The powder X-ray diffraction pattern of gained xinafoate mepivacaine is as attached figure 1 , the DSC diagram is attached figure 2 .
Embodiment 2
[0033] The preparation of embodiment 2 mepivacaine xinafoate
[0034] Mepivacaine base (1.23 g; 5 mmol) and xinafoic acid (0.94 g; 5 mmol) were added to DMSO (20 mL), and stirred at 50° C. for 4 hours. Subsequently, the reaction solution was added dropwise to 300 mL of water, and a white solid was precipitated, which was filtered and vacuum-dried to obtain mepivacaine xinafoate salt.
Embodiment 3
[0035] Embodiment 3 prepares the xinafoate mepivacaine salt of micron size
[0036] A jet mill (model JM-50A, Shanghai Minwang Machinery Technology Co., Ltd.) was used for jet micronization of mepivacaine xinafoate salt. The particle size distribution of mepivacaine xinafoate after airflow micronization was measured by a dry method using a Mastersizer 3000 laser scattering particle size distribution analyzer (Malvern Instrument, UK). The micronized mepivacaine xinafoate was determined to have the following particle size distribution: 10% < 2.16 μm, 50% < 4.21 μm and 90% < 7.85 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com