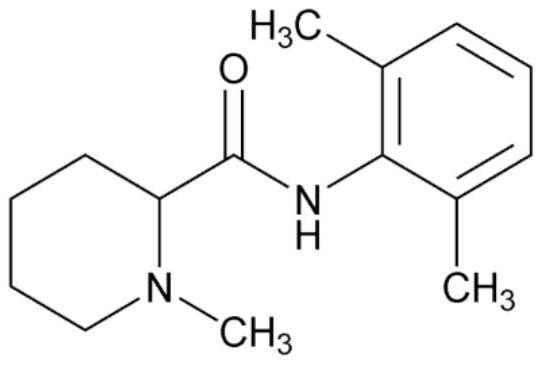
Mepivacaine xinafoate and its long-acting sustained-release preparation
A technology of mepivacaine xinafoate and mepivacaine xinafoate, which is applied in the field of medicine, can solve the problems of short duration of anesthesia, short biological half-life, high medical expenses, etc., to prolong anesthesia time, prolong half-life, and high safety sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 mepivacaine xinafoate
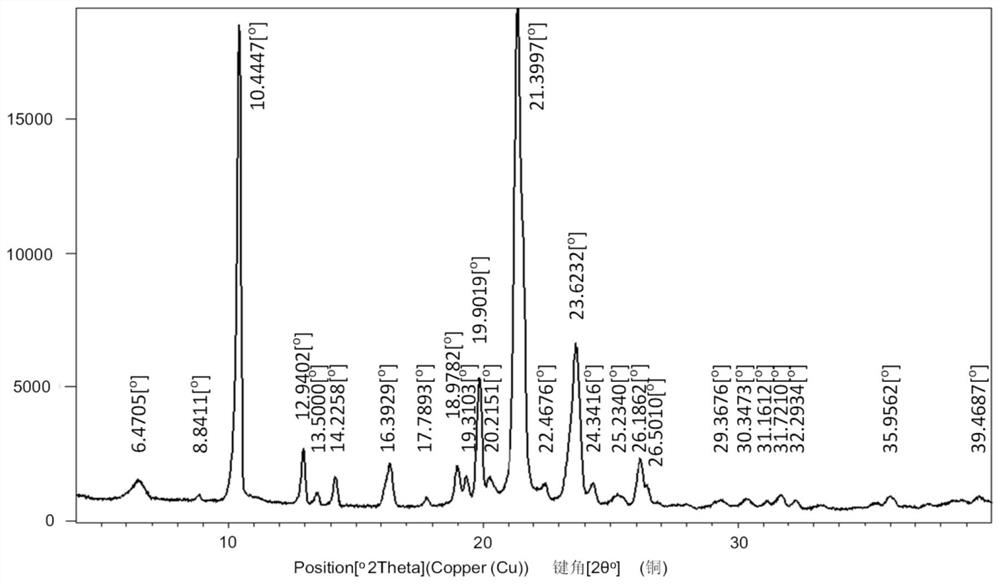
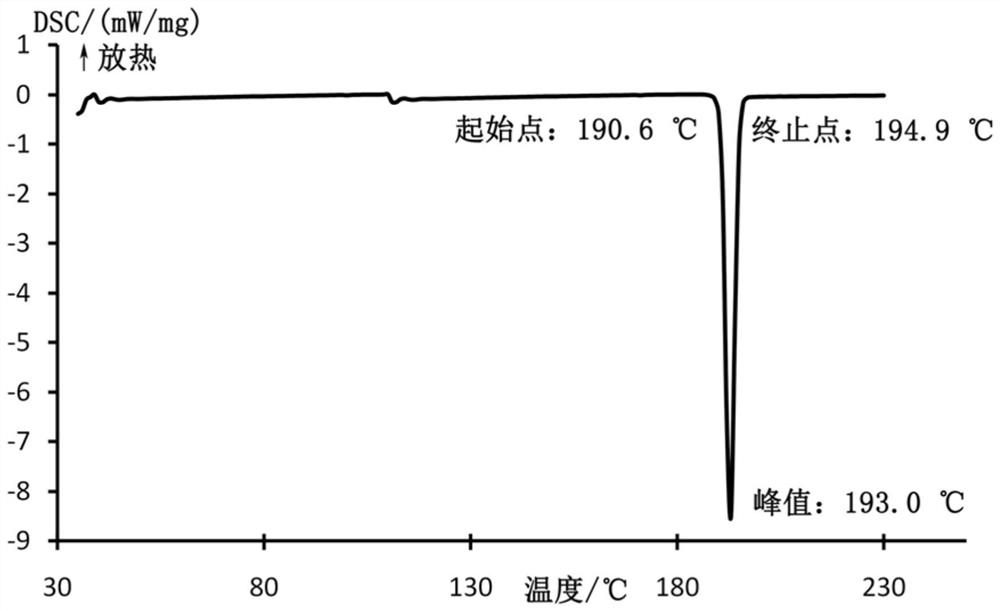
[0032] Mepivacaine base (12.3 g; 50 mmol) and xinafoic acid (9.41 g; 50 mmol) were added to absolute ethanol (300 mL) and stirred at 60°C for 5 hours. Subsequently, 2 L of water was added to the reaction solution to precipitate a white solid, which was filtered and dried in vacuo to obtain mepivacaine xinafoate salt. The powder X-ray diffraction pattern of the obtained mepivacaine xinafoate is as attached figure 1 , the DSC diagram is attached figure 2 .
Embodiment 2
[0033] The preparation of embodiment 2 mepivacaine xinafoate
[0034] Mepivacaine base (1.23 g; 5 mmol) and xinafoic acid (0.94 g; 5 mmol) were added to DMSO (20 mL) and stirred at 50°C for 4 hours. Subsequently, the reaction was added dropwise to 300 mL of water, and a white solid was precipitated, which was filtered and dried in vacuo to obtain mepivacaine xinafoate salt.
Embodiment 3
[0035] Example 3 Preparation of micron-sized mepivacaine xinafoate salt
[0036] The mepivacaine xinafoate salt was air-jet micronized using a jet mill (model JM-50A, Shanghai Minwang Machinery Technology Co., Ltd.). The particle size distribution of mepivacaine xinafoate after air flow micronization was determined by dry method using a Mastersizer 3000 laser scattering particle size distribution analyzer (Malvern Instrument, UK). Micronized mepivacaine xinafoate was determined to have the following particle size distribution: 10% < 2.16 μm, 50% < 4.21 μm and 90% < 7.85 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com