Recombinant novel coronavirus vaccine composition
A technology of vaccine composition and coronavirus, which is applied in the field of recombinant new coronavirus vaccine composition, can solve the problems of weak immunogenicity and achieve the effect of reducing viral load and good immune protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the preparation of vaccine composition
[0022] In order to study the technical effect of the vaccine composition provided by the present invention, the inventors have made the following vaccine composition preparations (0.5ml / dose), each composition contains rS protein, aluminum hydroxide adjuvant and / or CpG 7909. The specific preparation method is as follows: firstly, the CpG 7909 sample is adsorbed on the aluminum hydroxide adjuvant, and the adsorption samples of CpG 7909 / aluminum hydroxide adjuvant (w / w) with different ratios are prepared (here the content of the aluminum hydroxide adjuvant is substantially The above is the content of aluminum element); then add different concentrations of rS protein stock solution to the CpG 7909 / aluminum hydroxide adjuvant sample. After mixing the above formulations thoroughly, store at 2-8°C if not administered immediately. The specific ratio is shown in Table 1.
[0023] Table 1: Components of each vaccine comp...
Embodiment 2
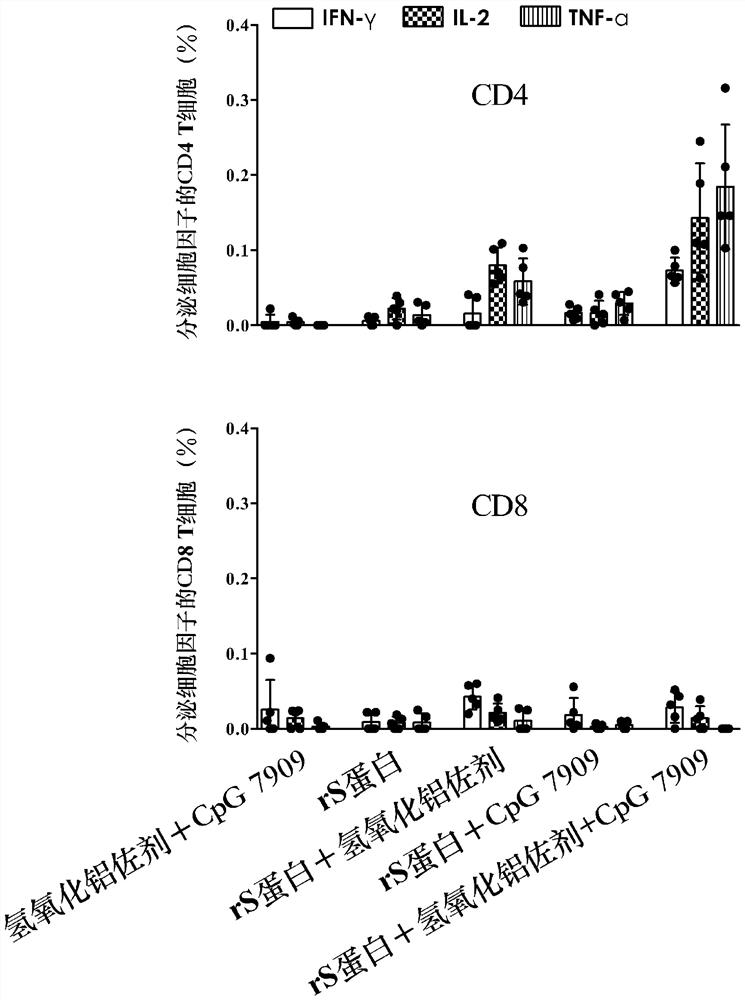
[0025] Example 2: Comparing the immunological evaluation of novel coronavirus vaccines with different adjuvant combinations on the BALB / c mouse model
[0026] 1. Grouping of mice and animal immunization strategy
[0027] Forty-eight SPF-grade female BALB / c mice (6-8 weeks old) were grouped. 8 or 10 mice in each group, 5 groups in total. For group information, see Table 2. The mice were immunized according to the grouping conditions in Table 2. The immunization method was intramuscular injection into the inner thigh of the mice, and the injection volume was 50 μl / dose / mouse (1 / 10 the intended dose for humans). Each mouse was immunized twice with an interval of 3 weeks.
[0028] Table 2: Comparison of grouping of BALB / c mice in experiments with different adjuvant combinations
[0029]
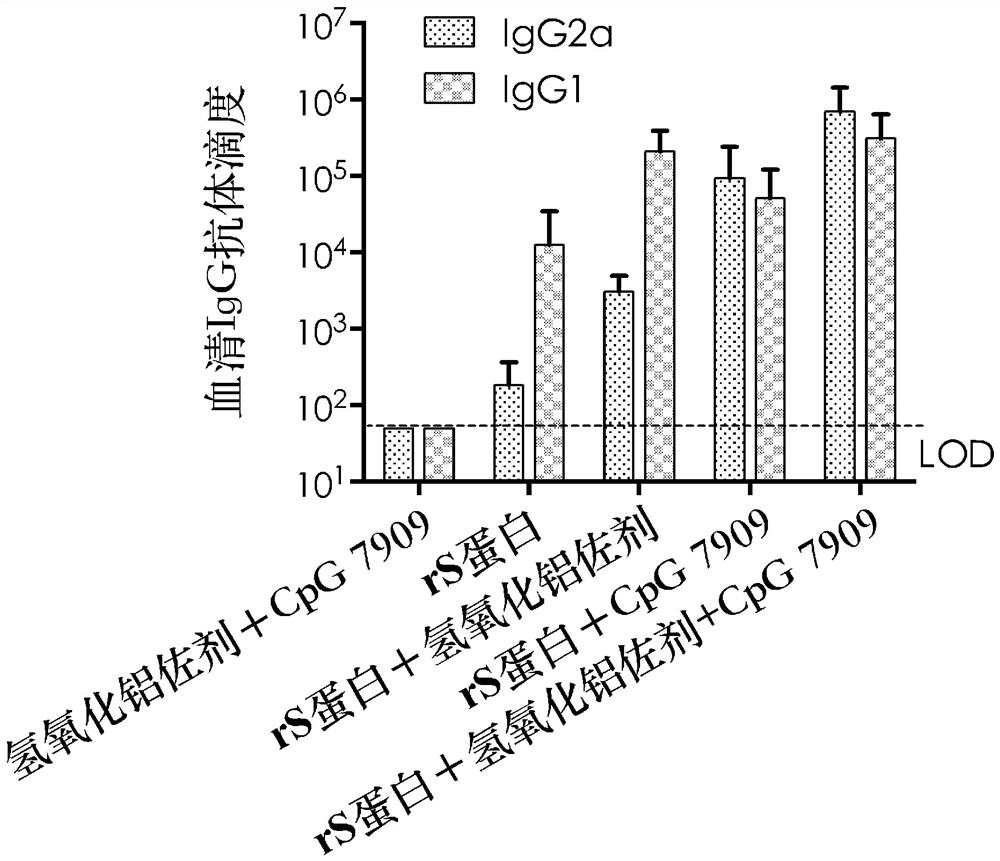
[0030] 2. Vaccine-Induced Antibody Response Detection
[0031] Two weeks after the second immunization, blood was collected from mice in each group, and serum was separated. IgG antibod...
Embodiment 3
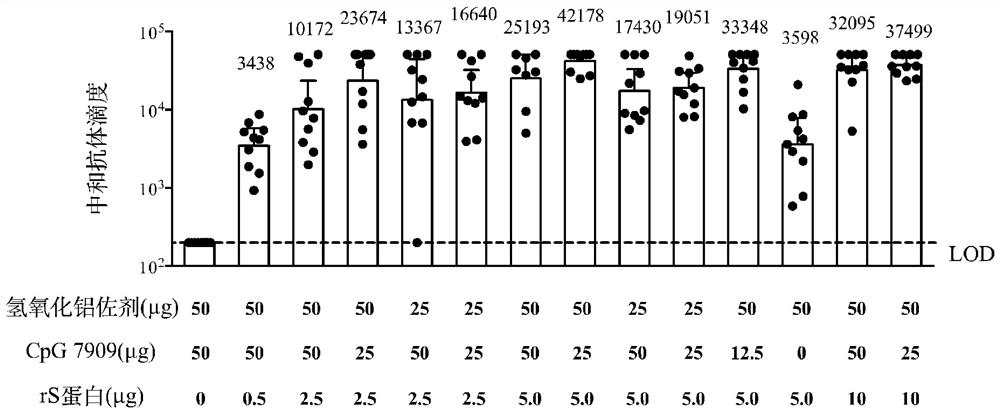
[0036] Example 3: Comparing the immunological evaluation of new coronavirus vaccines with different dosage ratios on the BALB / c mouse model
[0037] 1. Grouping of mice and animal immunization strategy
[0038] 140 SPF grade female BALB / c mice (6-8 weeks old) were grouped. 10 in each group, a total of 14 groups. For group information, see Table 3. The mice were immunized according to the grouping conditions in Table 3. The immunization method was intramuscular injection into the inner thigh of the mice, and the injection volume was 50 μl / dose / mouse (1 / 10 the intended dose for humans). Each mouse was immunized twice with an interval of 3 weeks.
[0039] Table 3: Grouping of BALB / c mice in comparative dose ratio experiments
[0040]
[0041] 2. Vaccine-Induced Antibody Response Detection
[0042] Two weeks after the second immunization, blood was collected from mice in each group, and serum was separated. IgG antibody titers against rS protein in serum were detected by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com