A kind of anti-h1n1 influenza pharmaceutical composition and application thereof
A composition and drug technology, applied in the direction of pharmaceutical formula, antiviral agents, plant raw materials, etc., can solve the problems of Tamiflu’s severe side effects, inapplicability to young people, and insufficient drug production capacity, so as to improve the survival rate and prolong the average survival time , Prevention and treatment of H1N1 infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] In addition, in order to facilitate the preparation of the pharmaceutical composition of the present invention into various medicaments more conveniently, the preparation method of the present invention also includes:
[0042] At 50-60°C, the filtrate obtained after ethanol extraction was concentrated into a thick paste.
[0043] Wherein, the relative density of the thick paste is preferably 1.10-1.15.
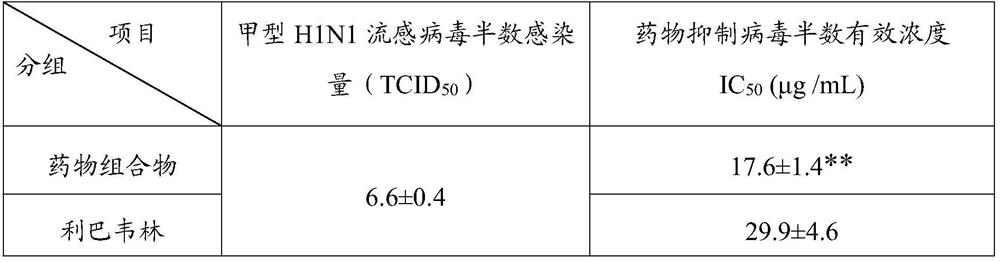
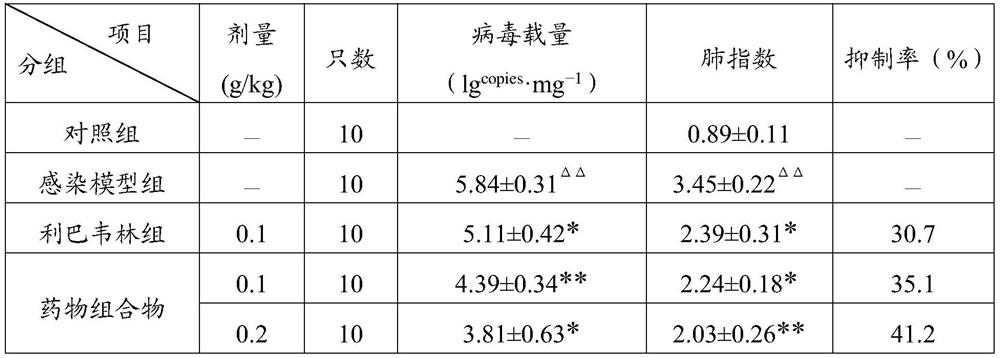
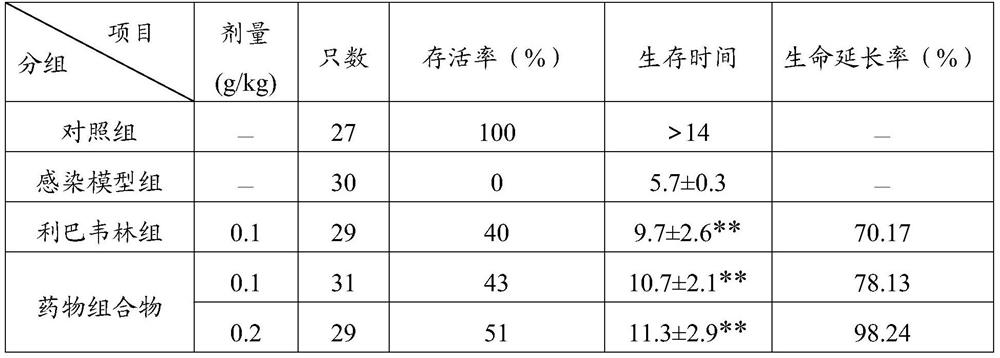
[0044] When the pharmaceutical composition of the present invention was compared with the inosine monophosphate dehydrogenase inhibitor ribavirin for inhibiting influenza A H1N1 virus in canine kidney MDCK cells in vitro, it was found that the inhibitory activity of the pharmaceutical composition was better than that of ribavirin. Bavirin; in the mouse nasal drop infection pneumonia test, the pharmaceutical composition of the present invention can significantly reduce the viral load and lung index of the lung tissue, and the inhibition rate of influenza A H1N1 influenza...
Embodiment 1
[0049] Example 1: Preparation of capsules of the pharmaceutical composition of the present invention
[0050] Take by weighing each crude drug (unit: g) by following weight: marine organism extract 30, Chinese medicine composition extract 80;
[0051] Get marine organisms (the proportion is 1 part of starfish and 6 parts of seaweed), the traditional Chinese medicine composition (the proportion is 7 parts of burdock, 4 parts of mulberry leaves, 5 parts of honeysuckle, 4 parts of forsythia, 6 parts of scutellaria baicalensis), add 7 parts respectively. 70% ethanol reflux extraction twice the total weight of the extract, 60min each time, filtered, the filtrate was concentrated under reduced pressure at 55 ° C to a thick paste with a relative density of 1.10-1.15, spray-dried to obtain the raw material drug (Marine Biologicals). extracts and traditional Chinese medicine composition extracts). The crude drug is weighed according to the above weight, sodium starch glycolate is adde...
Embodiment 2
[0052] Example 2: Preparation of tablets of the pharmaceutical composition of the present invention
[0053] Take by weighing each raw material medicine (unit: g) by following weight: marine organism extract 20, Chinese medicine composition extract 70;
[0054] Get marine organisms (the proportion is 1 part of starfish and 8 parts of seaweed), the traditional Chinese medicine composition (the proportion is 6 parts of burdock seed, 3 parts of mulberry leaf, 7 parts of honeysuckle, 2 parts of forsythia, 5 parts of skullcap), add 6 parts respectively. 90% ethanol twice the total weight of the extract was refluxed for 3 times, 60min each time, filtered, the filtrate was concentrated under reduced pressure at 50°C to a thick paste with a relative density of 1.10-1.15, and spray-dried to obtain the bulk drug. Weigh the crude drug according to the above weight, add sodium starch glycolate, mix well, and send it into a tablet press to form a tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com