HSV-2 DNA vaccine used by mucous membranes, as well as preparation method and application thereof
An HSV-2DNA, mucosal technology, applied in the fields of genetic engineering and immunology, can solve the problems of no ideal promotion method for vaccines, cumbersome operation, inconvenient use, etc., and achieve the effect of easy promotion and use, simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0035] 1. Amplification of the target gene:
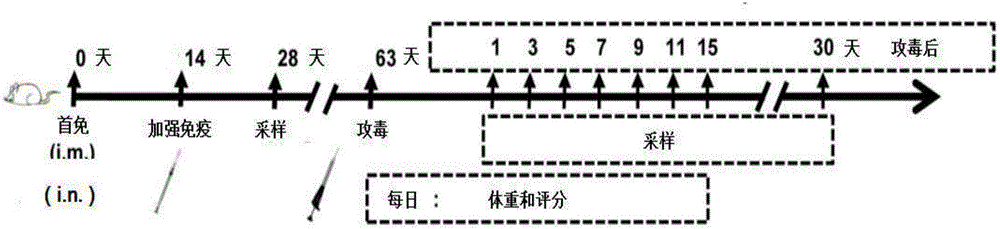
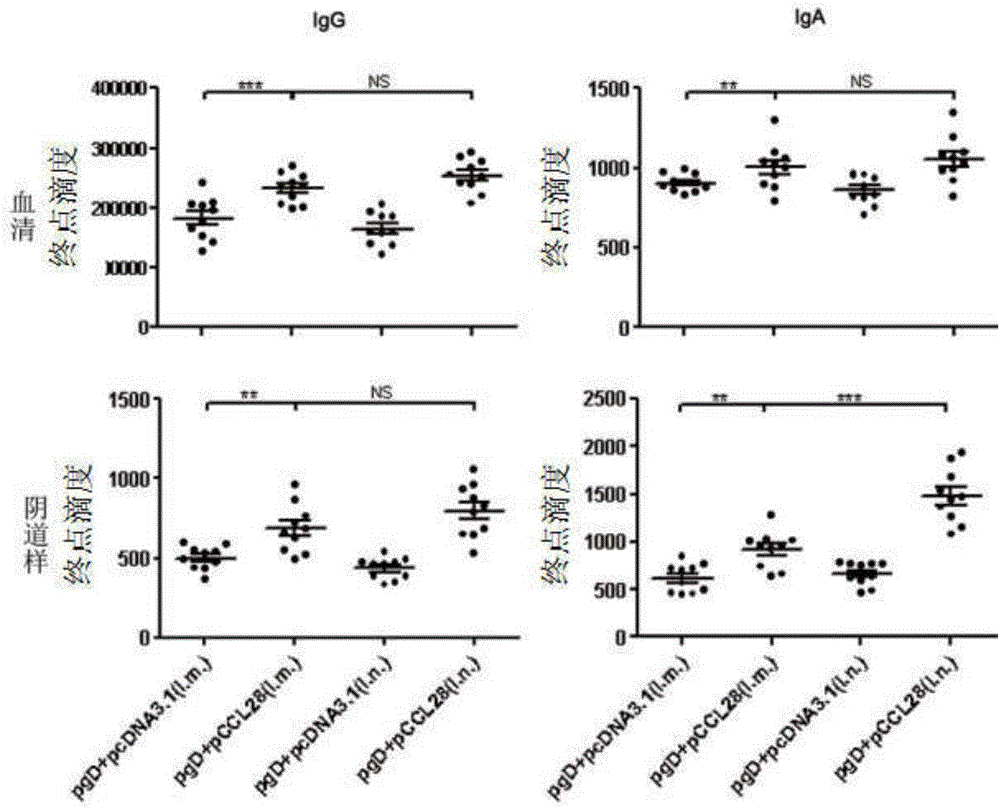
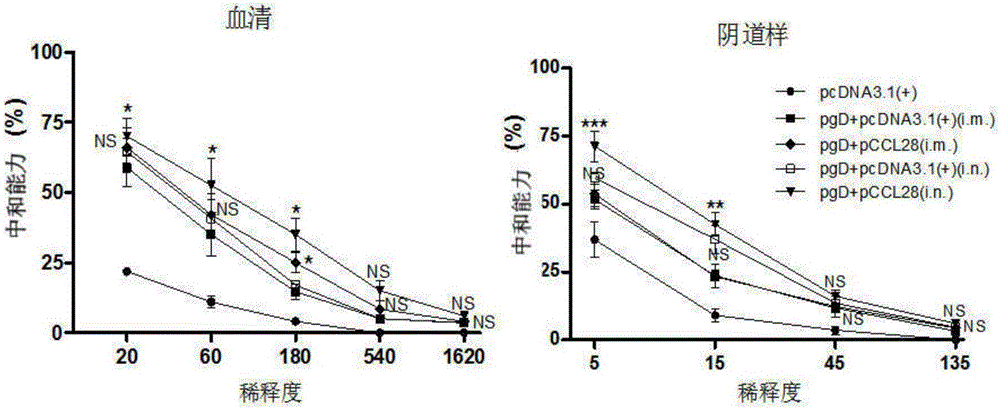
[0036] HSV-2 gD was successively constructed on the pcDNA3.1(+) plasmid vector (purchased from Invitrogen, the same below) (the experimental strain of HSV-2 was HG52 strain, purchased from LGC Pharmaceutical Impurity Standard Company, Royal Laboratory of England) (GenBank : Z86099.2) envelope glycoprotein gene and adjuvant gene CCL28 (Kai Hu. JOURNAL OF IMMUNOLOGY, Aug. 2013, p.1935-1947), the molecular formulas are: pcDNA3.1(+)-Xba I-gD- PmeI, pcDNA3.1 (+)-HindIII-CCL28-KpnI, named after pgD (for containing the pcDNA3.1 (+) plasmid of the nucleotide sequence shown in SEQ ID NO.1) and pCCL28 (for containing the nucleotide sequence shown in SEQ ID NO.1) respectively pcDNA3.1 (+) plasmid with the nucleotide sequence shown in NO.2), and the primer sequences for amplifying the target fragment are shown in Table 1.
[0037] Table 1 constructs the primer sequence list of pgD and pCCL28
[0038]
[0039] 2. Plasmid Construction
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com