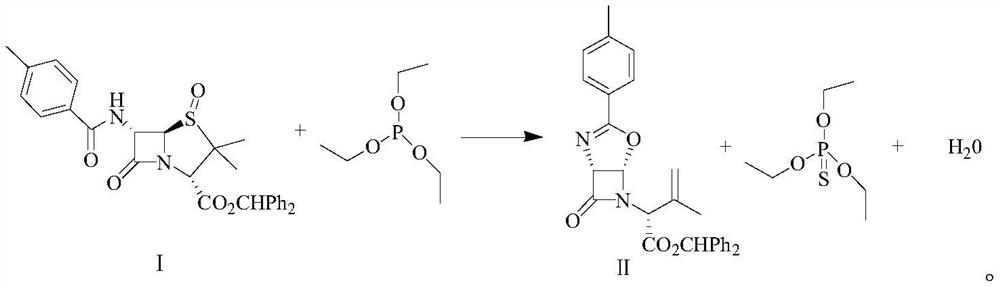
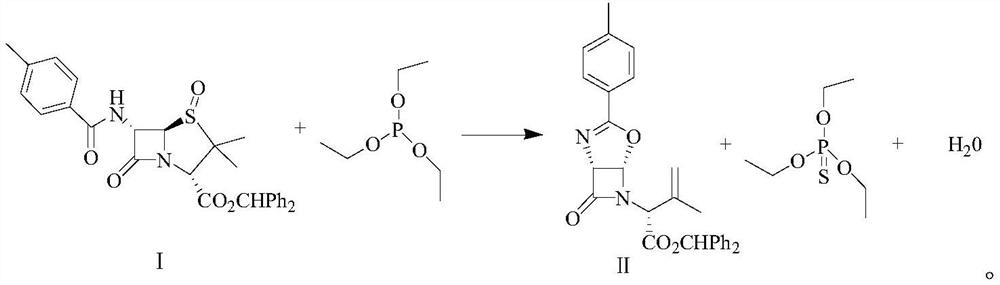
Synthetic method of latamoxef intermediate
A synthetic method, the technology of Latamoxef, which is applied in the field of synthesis of Latamoxef intermediates, can solve the problems of low yield, achieve the effects of reducing production costs, simplifying post-treatment procedures, and reducing the production of sulfur dioxide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] ① Add 370g of isobutanol to the three-necked flask, check that the solvent moisture is 0.05%, add 50g of 3,3-dimethyl-6-(4-methyl-benzamido)-4,7-dioxo-4 - benzhydryl thia-1-aza-bicyclo[3.2.0]heptane-2-carboxylate.
[0033] ②Stir and heat up to 50°C, and add 17g of triethyl phosphite.
[0034] ③Heating and reflux reaction, and the by-product water is separated under a slight negative pressure (-0.005MPa).
[0035] ④ It is detected that the residual raw material is 0.9%, and the isobutanol is distilled off after the reaction is completed.
[0036] ⑤ Add 200 g of acetone to the reaction liquid after steaming the isobutanol, control the temperature at 40° C., and grow the crystal for 4 hours.
[0037] ⑥ After the crystal growth is completed, cool down to 5°C, filter with suction, and wash the material with 65g of acetone.
[0038] ⑦The product (II) was obtained after vacuum drying at 35°C for 7 hours, with a molar yield of 78.12% and a purity of 98.85%.
[0039] ⑧Steam ...
Embodiment 2
[0041] ① Add 375g of isobutanol to the three-necked flask, check the solvent moisture to 0.06%, add 50g of 3,3-dimethyl-6-(4-methyl-benzamido)-4,7-dioxo-4 - benzhydryl thia-1-aza-bicyclo[3.2.0]heptane-2-carboxylate.
[0042] ②Stir and heat up to 47°C, and add 17.5g of triethyl phosphite.
[0043] ③Heating and reflux reaction, and the by-product water is separated out under a slight negative pressure (-0.008MPa).
[0044] ④ It is detected that the residual raw material is 0.85%. After the reaction is completed, the isobutanol is distilled off.
[0045] ⑤ Add 203 g of acetone to the reaction liquid after steaming the isobutanol, control the temperature at 35° C., and grow the crystal for 3 hours.
[0046] ⑥ After the crystal growth is completed, cool down to 2°C, filter with suction, and wash the material with 70g of acetone.
[0047] ⑦The product (II) was obtained after vacuum drying at 38°C for 6.5 hours, with a molar yield of 78.04% and a purity of 98.91%.
[0048] ⑧Steam...
Embodiment 3
[0050] ① Add 370g of isobutanol to the three-necked flask, check that the solvent moisture is 0.08%, add 50g of 3,3-dimethyl-6-(4-methyl-benzamido)-4,7-dioxo-4 - benzhydryl thia-1-aza-bicyclo[3.2.0]heptane-2-carboxylate.
[0051] ②Stir and heat up to 45°C, and add 17.5g of triethyl phosphite.
[0052] ③Heating and reflux reaction, and the by-product water is separated under a slight negative pressure (-0.01MPa).
[0053] ④ It is detected that the residue of the raw material is 0.92%. After the reaction is completed, the isobutanol is distilled off.
[0054] ⑤Add 205g of acetone to the reaction liquid after steaming isobutanol, control the temperature at 38°C, and grow the crystal for 3.5 hours.
[0055] ⑥ After the crystal growth is completed, cool down to 0°C, filter with suction, and wash the material with 70g of acetone.
[0056] ⑦ The product (II) was obtained after vacuum drying at 40°C for 6 hours, with a molar yield of 78.27% and a purity of 98.81%.
[0057] ⑧Steam ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com