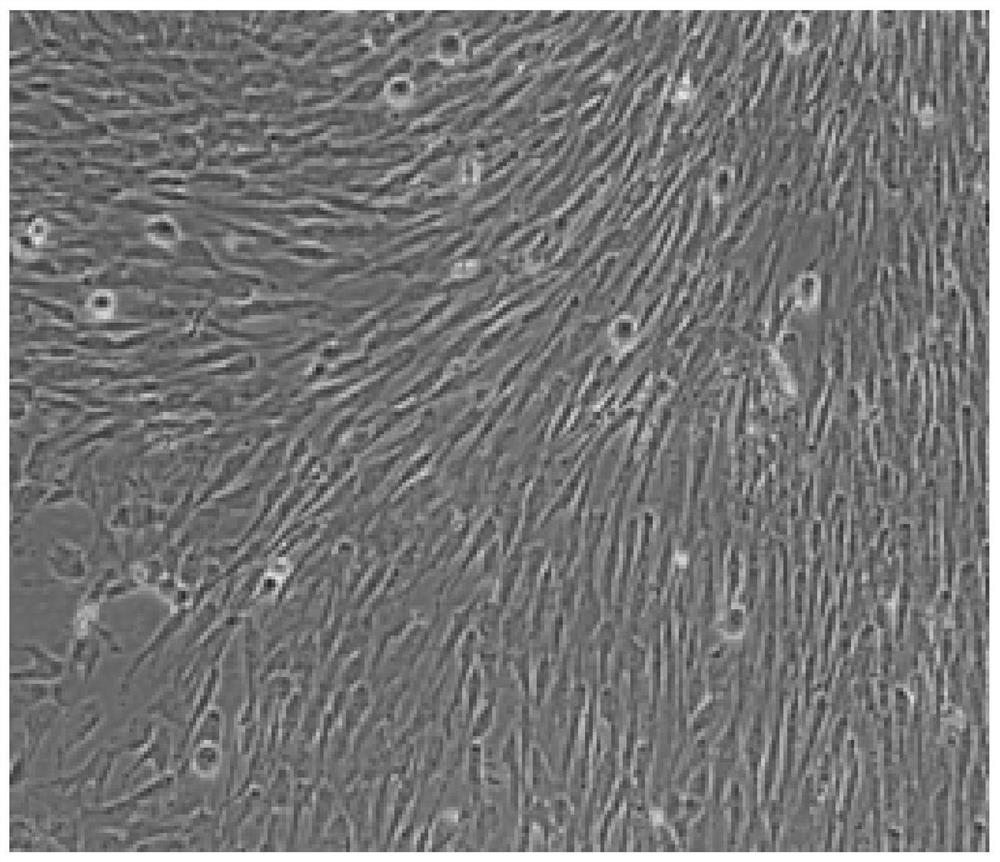
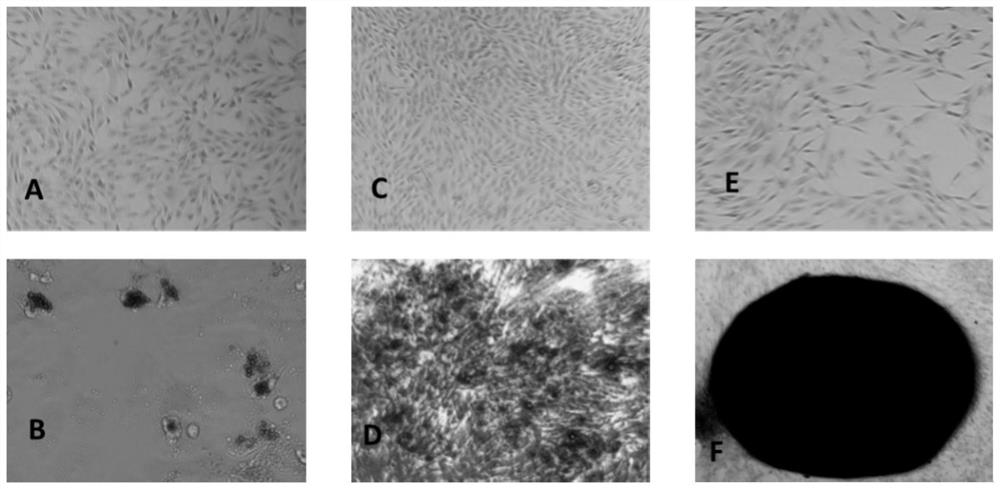
Method for preparing adipose tissue-derived stem cells by using serum-free culture medium
A kind of stem cell and culture medium technology, applied in cell dissociation method, biochemical equipment and method, culture process, etc., can solve the problem of unfavorable survival of stem cells and achieve excellent technical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1: Isolation and culture of primary adipose-derived mesenchymal stem cells
[0076] (1) The fat donated by volunteers (sample A) was processed in a biological safety cabinet and transported to the laboratory through a cold chain at 2-8°C;
[0077] (2) Centrifuge at 100xg for 5 minutes, remove the upper layer of adipose tissue, wash it again with D-Hanks solution, centrifuge at 100xg for 5 minutes, remove the adipose tissue, add 2 times the volume of 1% type Ⅱ collagenase, shake and digest for 30 minutes;
[0078] (3) After digestion, add double volume of D-hanks solution to dilute, centrifuge at 100xg for 5min, and keep the bottom cell pellet for the next operation;
[0079] (4) Take the cell pellet obtained in step (3), add the primary complete medium to resuspend, take a sample and count, according to 2×10 4 / cm 2 Inoculate into a T75 bottle; place in CO 2 Incubator (5% CO 2 , 37°C, saturated humidity);
[0080] (5) After culturing for 3 days, the cultu...
Embodiment 1a
[0082] Example 1a: Isolation and culture of primary adipose-derived mesenchymal stem cells
[0083] The operation and material of step (1) to step (3) are continued in embodiment 1;
[0084] (4) Take the cell pellet obtained in step (3) of Example 1, add the primary supplementary medium to resuspend, take a sample and count, according to 2×10 4 / cm 2 Inoculate into a T75 bottle; place in CO 2 Incubator (5% CO 2 , 37°C, saturated humidity);
[0085] (5) After culturing for 3 days, the culture medium was completely changed once, until (about 5 days) the cell confluence reached over 80%, the old medium was removed, the cells were washed with D-hanks solution, and 2ml of recombinant trypsin solution was added to each bottle to digest the cells The cells were detached for 2 minutes, diluted by adding 10ml of D-hanks solution to each bottle, centrifuged at 100xg for 10 minutes, and the cell pellet was resuspended in the primary supplemented medium to obtain primary adipose-der...
Embodiment 2
[0093] Example 2: Isolation and culture of primary adipose-derived mesenchymal stem cells
[0094] (1) The fat donated by volunteers (sample B) was processed in a biological safety cabinet and transported to the laboratory through a 2-8°C cold chain;
[0095] (2) Centrifuge at 100xg for 5 minutes, remove the upper layer of adipose tissue, wash it again with D-Hanks solution, centrifuge at 100xg for 5 minutes, remove the adipose tissue, add 2 times the volume of 1% type Ⅱ collagenase, shake and digest for 30 minutes;
[0096] (3) After digestion, add double volume of D-hanks solution to dilute, centrifuge at 100xg for 5min, and keep the bottom cell pellet for the next operation;
[0097] (4) Take the cell pellet obtained in step (3), add the primary complete medium to resuspend, take a sample and count, according to 2×10 4 / cm 2 Inoculate into a T75 bottle; place in CO 2 Incubator (5% CO 2 , 37°C, saturated humidity);
[0098] (5) After culturing for 3 days, the culture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com