Synthesis method and application of o-vanillin Schiff base platinum complex
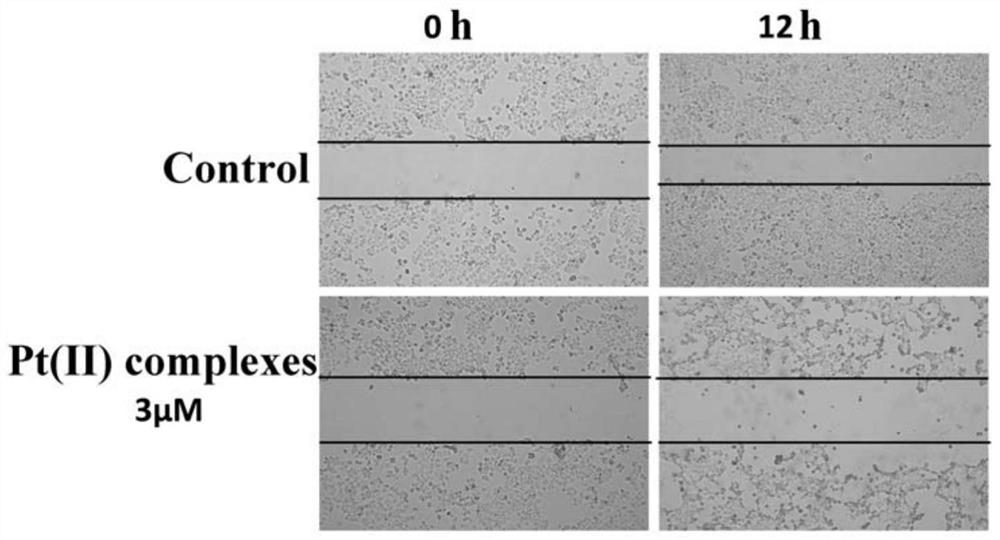
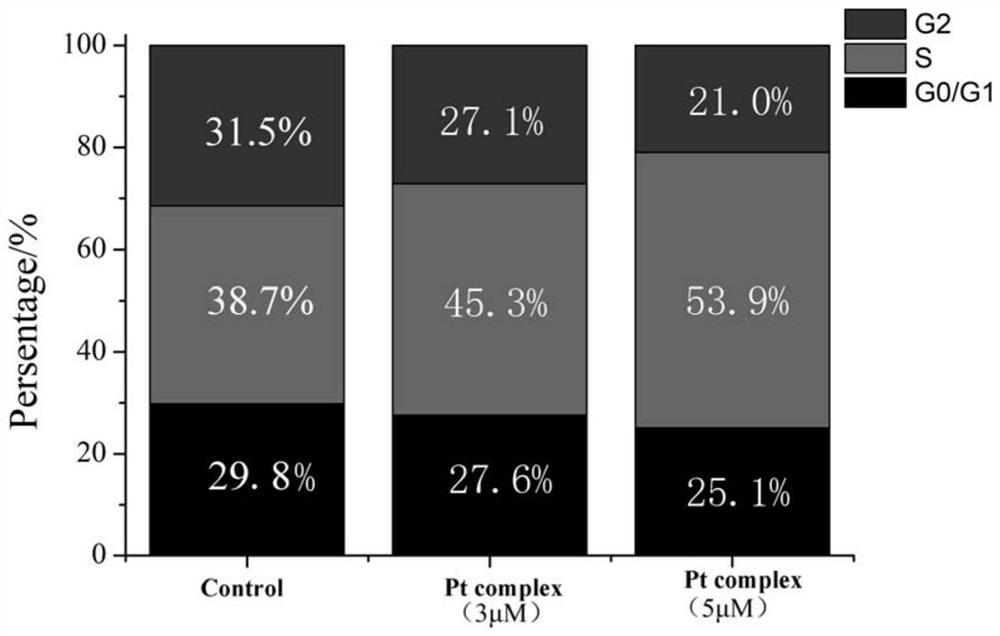
A technology of o-vanillin and Schiff base platinum, which is applied in the field of synthesis of o-vanillin Schiff base platinum complexes, and can solve problems such as drug resistance, organ toxicity and side effects, and severe side effects of platinum anticancer drugs , to achieve the effect of blocking cell replication, strong activity, and inhibiting cell migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
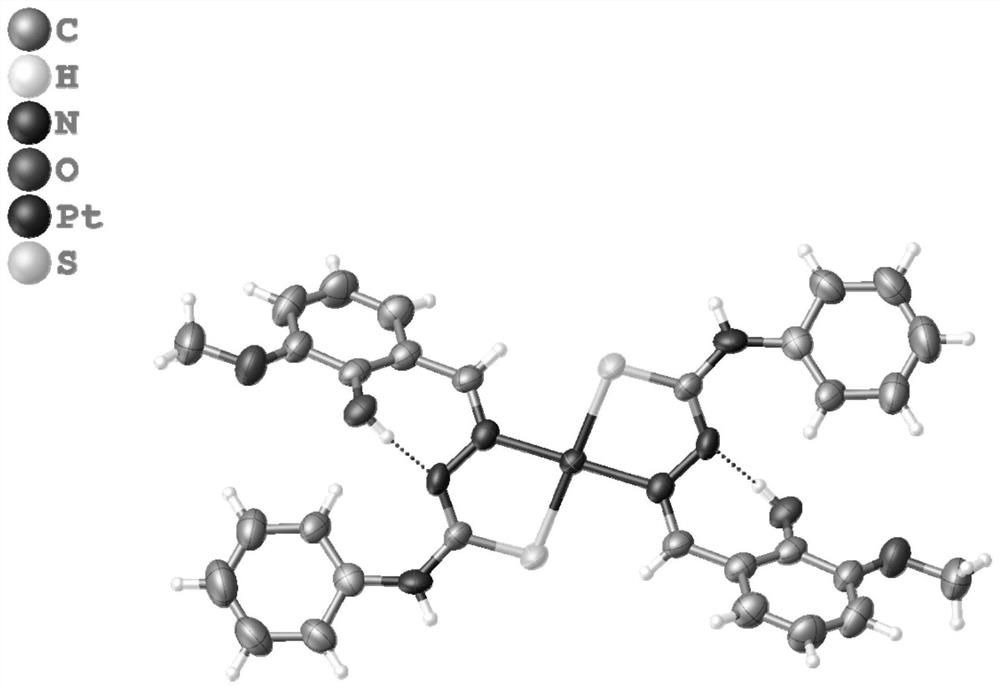
[0038] A method for synthesizing o-vanillin Schiff base platinum complexes, that is, synthesizing o-vanillin condensed 4-phenyl-3-thiosemicarbazide platinum complexes. The reaction route of the synthesis method is as follows:
[0039]
[0040] Concrete reaction steps are as follows:
[0041] 1) Dissolve 10mmol of o-vanillin and 10mmol of 4-phenyl-3-thiosemicarbazide in 50mL of methanol, and stir until dissolved;
[0042] 2) The above mixed solution was refluxed and stirred at 70°C for 6 hours to obtain a white solution;
[0043] 3) The white solution was rotary evaporated under reduced pressure to obtain a white powder, washed three times with a small amount of methanol, and dried to obtain the ligand, yield: 93.0%, mass spectrum, ESI+m / z: C 15 h 15 N 3 o 2 S, 301.09 [M] + ;
[0044] 4) Take a test tube, weigh 0.1mmol ligand and 0.05mmol K 2 PtCl 4 , continue to add 5mL methanol, seal;
[0045] 5) After heating in an oven at 70°C for 4 hours, golden yellow crystals...
Embodiment 2
[0053] A method for synthesizing o-vanillin Schiff base platinum complexes, i.e. synthesizing o-vanillin condensed 4-phenyl-3-thiosemicarbazide platinum complexes. The reaction route of the synthesis method is the same as in Example 1, and the specific reaction steps are as follows :
[0054] 1) Dissolve 8 mmol of o-vanillin and 12 mmol of 4-phenyl-3-thiosemicarbazide in 45 mL of methanol, and stir until dissolved;
[0055] 2) The above mixed solution was refluxed and stirred at 65°C for 8 hours to obtain a white solution;
[0056] 3) The white solution was rotary evaporated under reduced pressure to obtain a white powder, washed three times with a small amount of methanol, and dried to obtain the ligand, yield: 90.3%, mass spectrum, ESI+m / z: C 15 h 15 N 3 o 2 S, 301.09 [M] + ;
[0057] 4) Take a test tube, weigh 0.08mmol ligand and 0.06mmol K 2 PtCl 4 , continue to add 4mL methanol, seal;
[0058] 5) After heating in an oven at 65°C for 6 hours, golden yellow crystal...
Embodiment 3
[0061] A method for synthesizing o-vanillin Schiff base platinum complexes, i.e. synthesizing o-vanillin condensed 4-phenyl-3-thiosemicarbazide platinum complexes. The reaction route of the synthesis method is the same as in Example 1, and the specific reaction steps are as follows :
[0062] 1) Dissolve 12mmol of o-vanillin and 8mmol of 4-phenyl-3-thiosemicarbazide in 55mL of methanol, and stir until dissolved;
[0063] 2) The above mixed solution was refluxed and stirred at 75°C for 4 hours to obtain a white solution;
[0064] 3) The white solution was rotary evaporated under reduced pressure to obtain a white powder, washed three times with a small amount of methanol, and dried to obtain the ligand. The yield: 89.7%, mass spectrometry, ESI+m / z: C 15 h 15 N 3 o 2 S, 301.09 [M] + ;
[0065] 4) Take a test tube, weigh 0.12mmol ligand and 0.04mmol K 2 PtCl 4 , continue to add 6mL methanol, seal;
[0066] 5) After heating in an oven at 75°C for 5 hours, golden yellow cr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com