Ortho-pyridine hydrazide derivatives and their production methods, pharmaceutical compositions and uses
A technology of hydrazide derivatives and urea derivatives, applied in the fields of o-pyridine hydrazide derivatives and their preparation methods, pharmaceutical compositions and uses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0054] Embodiment 1.4-Chloro-N-propylidene picoline hydrazide
[0055]
[0056] Add 1.0 g of 4-chloropicoline hydrazide to the flask, add 20 ml of acetone to dissolve it, add 1 drop of glacial acetic acid, stir and react at room temperature for 48 hours, TLC monitors the end of the reaction, concentrate the reaction solution, and add 30 ml of acetic acid Ethyl ester was dissolved, washed with saturated sodium bicarbonate solution, dried over anhydrous sodium sulfate, and evaporated to dryness to obtain 0.9 g of white solid. 1 H NMR (300MHz) (CDCl 3 ), 10.56 (S, 1H, CONH), 8.45 (d, 1H, ArH), 8.31 (S, 1H, ArH), 7.46 (d, 1H, ArH), 2.17 (S, 1H, CH 3 ), 2.04 (S, 1H, CH 3 ).MS(FAB):(M + +1=212).
Embodiment 24
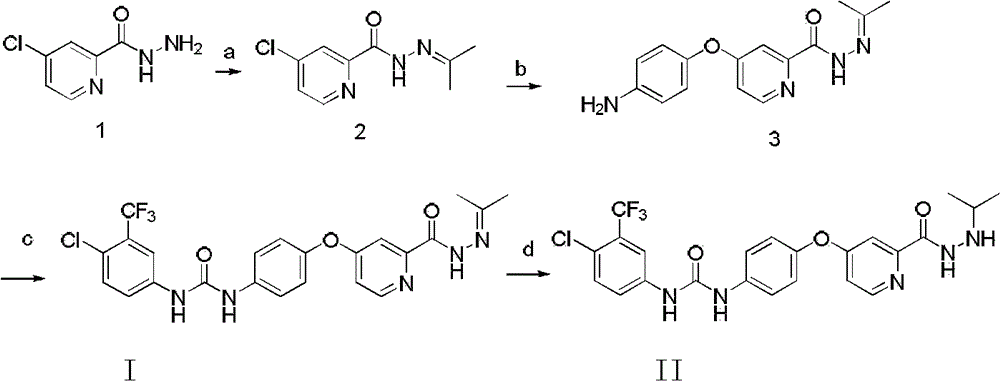
[0057] Example 2.4-p-aminophenoxy-N-propylidene picoline hydrazide
[0058]
[0059] Dissolve 2.46 g of p-aminophenol in 20 ml of DMF at room temperature, add 2.6 g of potassium tert-butoxide, stir for 2 hours, add 3.0 compound and 0.09 g of K2C03 and heat at 80 ° C for 16 h under nitrogen protection. After the reaction, cool to room temperature, evaporate DMF to dryness, add 10ml ethyl acetate and 10ml distilled water for extraction, extract the aqueous layer three times with 10ml ethyl acetate, combine the organic layers, wash three times with 50ml saturated brine, and dry over anhydrous Na2SO4. A purple solid was separated by column chromatography, 1 H NMR (300MHz)(DMSO-d6), 10.69(S, 1H, CONH), 8.47(d, 1H, ArH), 7.37(S, 1H, ArH), 7.11(d, 1H, ArH), 6.87(d , 2H, ArH), 6.65 (d, 2H, ArH), 5.18 (S, 2H, NH 2 ), 2.00 (S, 1H, CH 3 ), 1.93 (S, 1H, CH 3 )., MS(FAB)(M + +1=244)
Embodiment 3
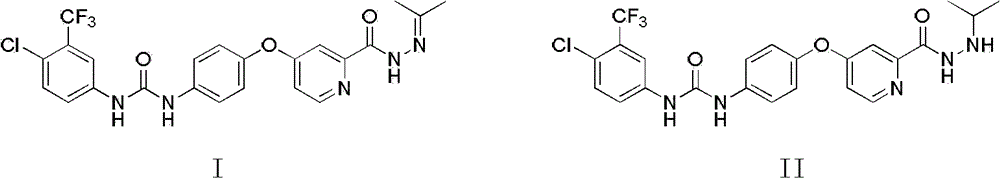
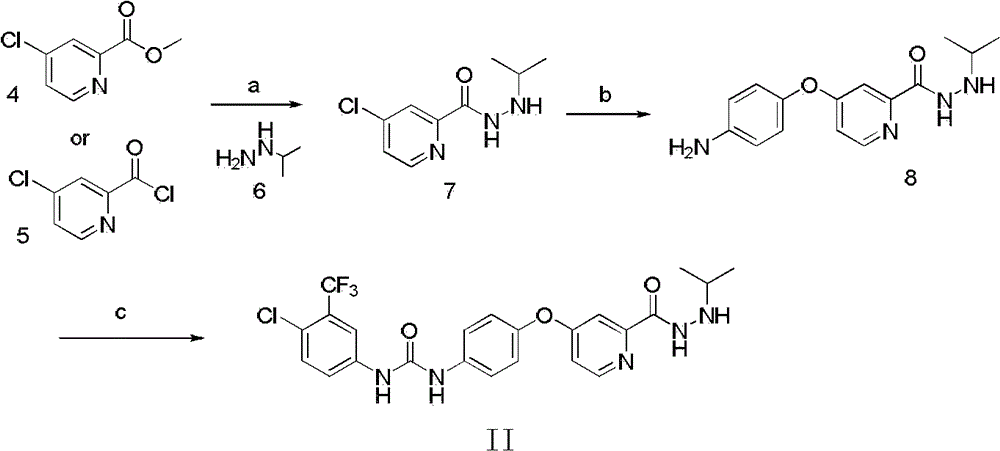
[0060] Example 3.1-p-Chlorotrifluoromethylphenyl-3-(4-(2-propylidene hydrazinoylpyridine-4-oxy)phenyl)urea
[0061]
[0062] 6.076 g of CDI was dissolved in 20 mL of dichloromethane, and a catalytic amount of DMAP was added. Then add dropwise 6.642 g of p-chlorotrifluoromethylaniline in 60 ml of dichloromethane solution at room temperature. After the addition, stir at room temperature for 5 hours. After TLC monitors the disappearance of the raw material, add dropwise 4-p-aminophenoxy-N-propane After the addition of the dichloromethane solution of forked picoline hydrazide, the temperature was raised to 50° C. and the reaction was heated for 7 hours to stop the reaction. The solvent was removed under reduced pressure, and 4.0 g of the product was obtained by column chromatography.
[0063] 1 H NMR (300MHz) (DMSO-d6), 10.72 (S, 1H, NH), 9.23 (S, 1H, NH), 9.02 (S, 1H, NH), 8.54 (d, 1H, ArH), 8.11 (S , 1H, ArH), 7.50-7.75 (m, 4H, ArH), 7.40 (S, 1H, ArH), 7.13-7.28 (m, 3H, ArH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com