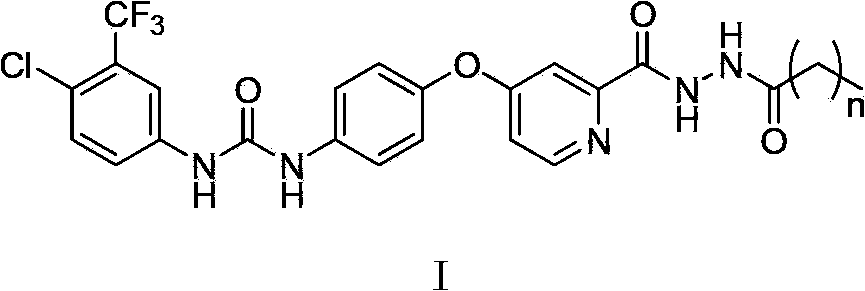
N'-straight chain alkylacyl o-pyridine hydrazide derivative, its preparation method, medicinal composition and use
A technology of straight-chain alkanoyl-o-pyridine hydrazides, which is applied in drug combinations, active ingredients of heterocyclic compounds, antineoplastic drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1.1-(4-chloro-3-trifluoromethylphenyl)-3-(4-(2-(2-(n-butyryl)hydrazinecarbonyl)pyridine-4-oxyl)phenyl)urea
[0056]
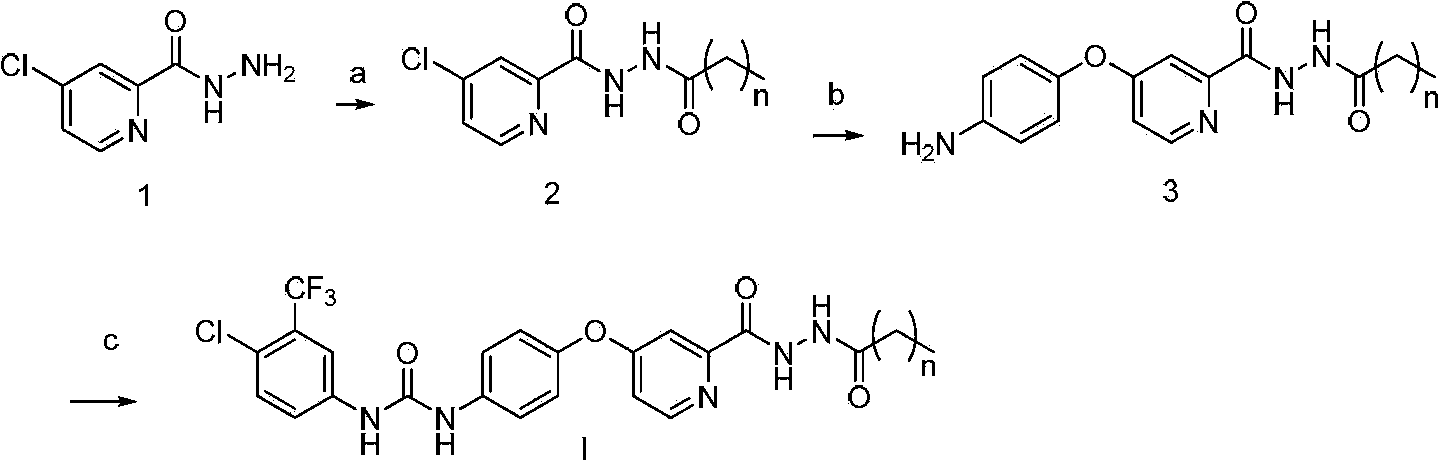
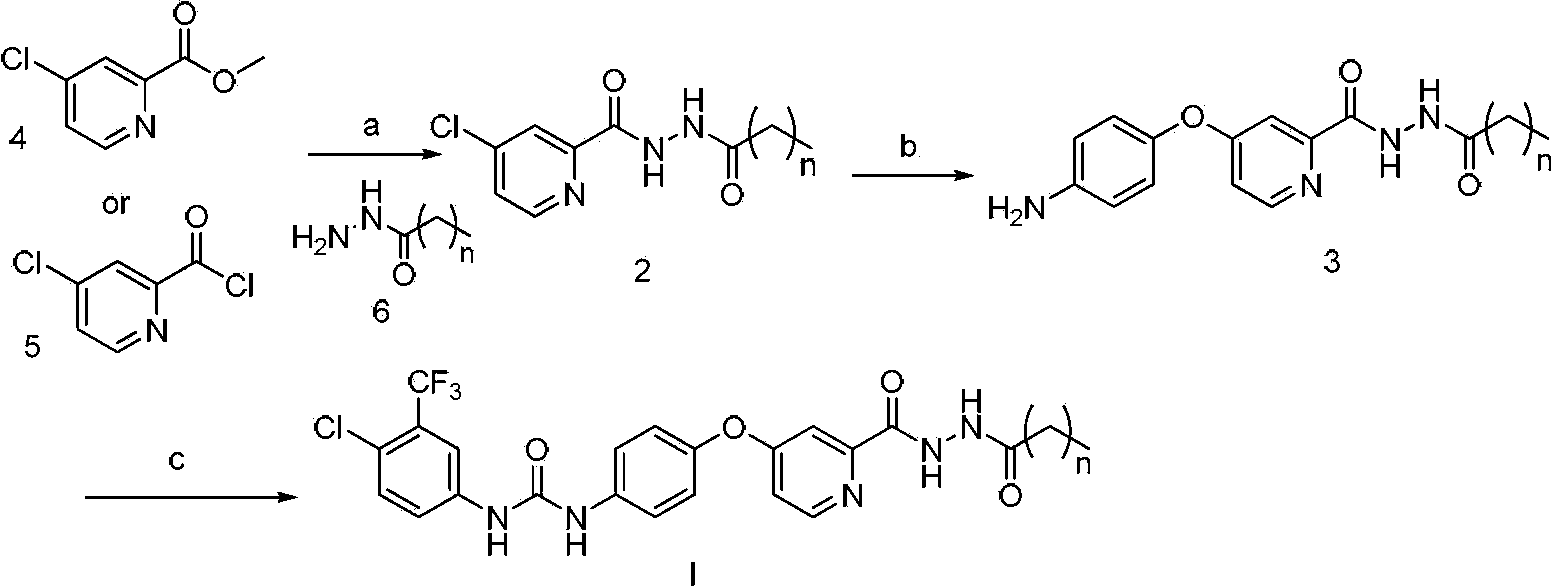
[0057] Synthesis of N'-n-butyryl-4-chloropyridine-2-hydrazide
[0058] Dissolve 0.6g (7.0mmol) of butyric acid in 3mL of DMF, add 2.1g (5.8mmol) of raw materials 4-chloropyridine-2-hydrazide, 2.7g (7.0mmol) of HATU, 1.8g (17.4mmol) of triethylamine, Stir at room temperature, TLC monitors that the reaction of raw materials is complete, add 100mL of water, a large amount of off-white solid precipitates, and 1.39g of the product is obtained. 1 H NMR (400MHz, DMSO-d 6 ):10.51(s,1H,-CONH-),9.98(s,1H,-CONH-),8.66(d,1H,Ar-H),8.03(d,1H,Ar-H),7.81(dd, 1H, Hz, Ar-H), 2.16(t, 2H, -COCH 2 -),1.56(m,2H,-CH 2 -Me),0.92(t,3H,-CH 3 ).MS(FAB):(M + +1=242).
[0059] Synthesis of N'-n-butyryl-4-(p-aminophenoxy)pyridine-2-hydrazide
[0060] Dissolve 295 mg (2.7 mmol) of p-aminophenol in 5 mL of DMF, add 416 mg (3.4 mmol) of potassium tert-butoxide under n...
Embodiment 2
[0065] Example 2.1-(4-(2-(2-acetylhydrazidecarbonyl)pyridine-4-oxyl)phenyl)-3-(4-chloro-3-trifluoromethylphenyl)urea
[0066]
[0067] Utilizing acetic acid instead of n-butyric acid, referring to the operation of Example 1, 175 mg of the target compound was obtained as a white solid. 1 HNMR (300MHz, DMSO-d 6 ):δ(ppm):10.38(s,1H,-NHCO-CH 3 ),9.99(s,1H,-NHCO-),9.21(s,1H,-NHCO-),9.00(s,1H,-NHCO-),8.53(d,1H,ArH),8.11(s,1H, ArH),7.67~7.62(m,2H,ArH),7.59(d,2H,ArH),7.36(d,1H,ArH),7.20~7.17(m,3H,ArH),1.88(s,3H,- CH 3 ).MS(FAB)(M + +1=508)
Embodiment 3
[0068] Example 3.1-(4-(2-(2-propionylhydrazidecarbonyl)pyridine-4-oxyl)phenyl)-3-(4-chloro-3-trifluoromethylphenyl)urea
[0069]
[0070] Using n-propionic acid instead of n-butyric acid, refer to the operation of Example 1 to obtain 135 mg of the target compound as a white solid. 1 H NMR (400MHz, DMSO-d 6 ):δ(ppm):10.37(s,1H,-NHCO-CH 2 -),9.94(s,1H,-NHCO-),9.22(s,1H,-NHCO-),9.01(s,1H,-NHCO-),8.55(d,1H,ArH),8.12(s,1H ,ArH),7.68~7.63(m,2H,ArH),7.60(d,2H,ArH),7.37(d,1H,ArH),7.20~7.18(m,3H,ArH),2.16(q,2H, -CH 2 -),1.04(t,3H,-CH 3 ).MS(FAB)(M + +1=522)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com