Antibody variable domains targeting the nkg2d receptor
A structural domain and variable technology, applied in the direction of antibody, receptor/cell surface antigen/cell surface determinant, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve problems such as adverse side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0181] Example 1 - Binding affinity of various NKG2D binding domains
[0182] The kinetics and affinities of various NKG2D binding domains were assessed by surface plasmon resonance using a Biacore 8K instrument (GE Healthcare). Anti-human Fc antibodies were immobilized on CM5 chips using standard amine coupling chemistry. Human monoclonal antibodies containing various NKG2D binding domains were captured on an anti-human Fc chip at a density of approximately 100 RU. A solution containing 0.411-100 nM soluble mouse Fc-human NKG2D dimer was injected at 30 μl / min at 37°C onto the captured NKG2D antibody and control surfaces. Surfaces were regenerated between cycles by rapid injection of 10 mM glycine pH 1.8. To obtain kinetic rate constants, bi-reference data were fitted to a 1:1 interaction model using Biacore 8K evaluation software (GE Healthcare). Equilibrium binding constant K D by the dissociation constant k d with the association constant k a ratio (k d / k a )Sure. ...
Embodiment 2
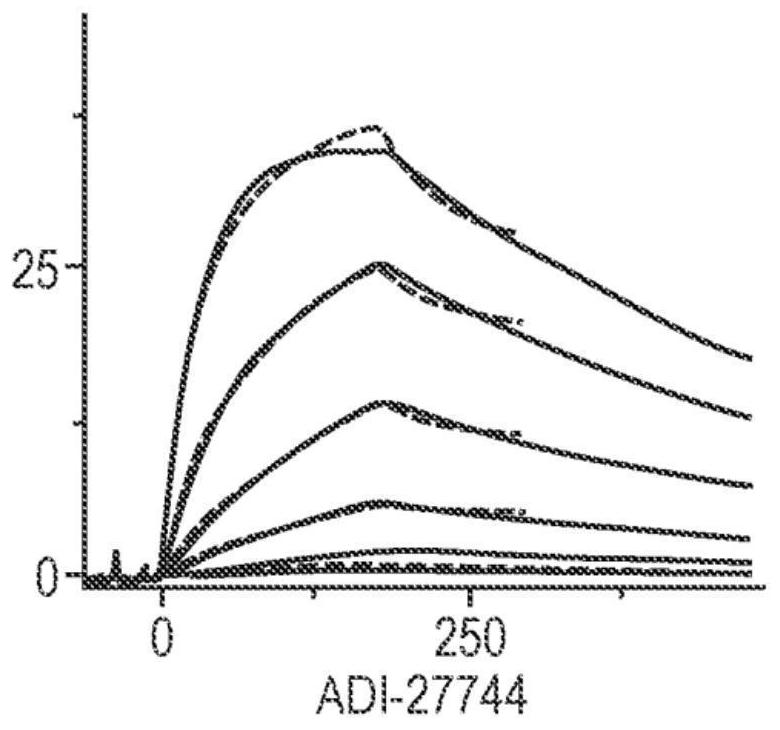
[0184] Example 2 - Binding epitope clustering of ADI-27744 clones
[0185] Clustering of ADI-27744(A44) NKG2D-binding domains was performed by surface plasmon resonance on a series of antibodies and ULBP6, the natural ligand for NKG2D, using a Biacore 8K instrument. Briefly, mouse Fc-human NKG2D was captured at a density of approximately 100 RU using an anti-mouse Fc antibody immobilized on a CM5 chip. Antibodies including ADI-27744, ADI-27749, F47 (sequence listed below) or 1D11 (commercial monoclonal NKG2D antibody), ULBP6 (sequence listed below), NKG2D monoclonal antibodies of MS (NKG2D antibody from Novo Nordisk, sequence listed below) and MAB139 (NKG2D antibody from R&D system, clone 149810). Biacore 8K evaluation software was used for all data analysis.
[0186] Table 10
[0187]
[0188]
[0189] ULBP amino acid sequence SEQ ID NO:67:
[0190]
[0191] Figure 4A A profile of NKG2D monoclonal antibody containing ADI-27744 injected on immobilized NKG2D foll...
Embodiment 3- 3
[0193] Example 3 - Trispecific Binding Protein Binding to NKG2D
[0194] The EL4 mouse lymphoma cell line was engineered to express human NKG2D. detect as figure 1 Shown is the effect of trispecific binding proteins (TriNKETs) each containing an NKG2D binding domain, a tumor-associated antigen binding domain (such as a CD33 or HER2 binding domain), and a CD16-binding Fc domain on extracellular NKG2D expressed on EL4 cells. affinity. Binding of multispecific binding proteins to NKG2D was detected using a fluorophore-conjugated anti-human IgG secondary antibody. Cells were analyzed by flow cytometry and fold-over-background (FOB) was calculated using mean fluorescence intensity (MFI) of NKG2D-expressing cells compared to parental EL4 cells.
[0195] TriNKETs tested included CD33-TriNKET-A44 (ADI-27744 and CD33 binding domain), CD33-TriNKET-A49 (ADI-27749 and CD33 binding domain), CD33-TriNKET-F63 (ADI-29463 and CD33 binding domain ), HER2-TriNKET-A44 (ADI-27744 and CD33 bind...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com