Gene therapy for CNS degeneration
A kind of therapy, transgenic technology, applied in the field of gene therapy of CNS degeneration, can solve few problems such as gene therapy of central nervous system degeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0379] Evaluation of Parkin transgenic variants
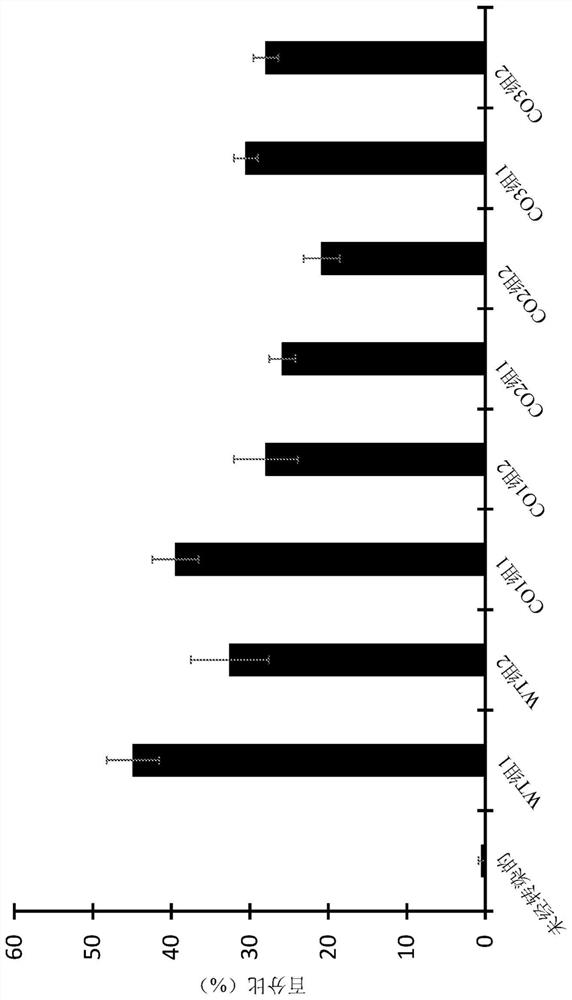
[0380] A series of plasmid vectors was generated to evaluate the expression of Parkin transgenic variants. expression cassette ( figure 1 ) contains in 5' to 3' order the CMV immediate early (IE) enhancer / promoter and 5'UTR, Parkin transgene 2A linked to enhanced green fluorescent protein (eGFP), 3'UTR and rabbit globin polyadenylation Acidifying sequence (polyA). The wild-type sequence (WT) or one of four codon-optimized variants (CO1 to CO4) of human PRKN were tested against the Parkin transgene. The sequences of wild-type and codon-optimized Parkin transgenes are provided in SEQ ID NO:27, 35, 36, 37 and 38.
[0381] One consideration in the design of codon-optimized CO1 to CO4 is the number of CpG sites. A CpG island is a region of DNA in which cytosine nucleotides are followed by guanine nucleotides in a linear sequence of bases along its 5'→3' direction. CpG islands (which are generally defined as polynucleotide sequ...
example 2
[0389] Selection of CO4 Parkin Transgene Expression Cassette
[0390] To assess other regulatory elements, various AAV expression cassettes were constructed as transfer plasmids for use in the helper-free AAV packaging system. AAV expression cassette ( Figure 7 ) contains in 5' to 3' order the 5'ITR of AAV2, the CMV enhancer (Enh) or no enhancer, a promoter selected from Table 5, a 5' untranslated region (UTR) selected from Table 6, a Parkin transgene Variant CO4 (SEQ ID NO:38), 3' untranslated region selected from Table 7, polyadenylation sequence (polyA) selected from Table 8, and 3'ITR of AAV2. A diagram of the box and various components is provided at Figure 8 middle. For detection of transgene expression in in vitro assays, the polynucleotide sequence (SEQ ID NO:80) encoding the N-terminal FLAG / HA-tag (SEQ ID NO:81) is in each of the sequences listed in Table 10 Inserted after the start codon on the Parkin transgene.
[0391] SH-SY5Y cells were cultured on 24-well ...
example 3
[0400] In vivo and clinical testing of Parkin AAV gene therapy
[0401] AAV vectors were generated by packaging the AAV vector genomes from constructs 1 to 20 in Example 2, specifically constructs 1, 7, 11 and 15 from Table 11. Testing is performed in rats, and one or more constructs are selected for testing in non-human primates (NHPs). Dose finding studies are performed and the starting dose for clinical trials is determined by measuring protein expression and observed toxicity.
[0402] The clinical trial was conducted in subjects identified as having a recessive mutation in the PRKN gene (also known as PRK2). Protein expression and observed toxicity were used to determine optimal doses. Efficacy was assessed using a modification of the Unified Parkinson's Disease Rating Scale (UPDRS).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com