Triterpenes in leaves of Phyllanthus acidus as well as pharmaceutical composition and application of triterpenes
A technology of triterpene compounds and compositions, applied in the field of triterpene compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
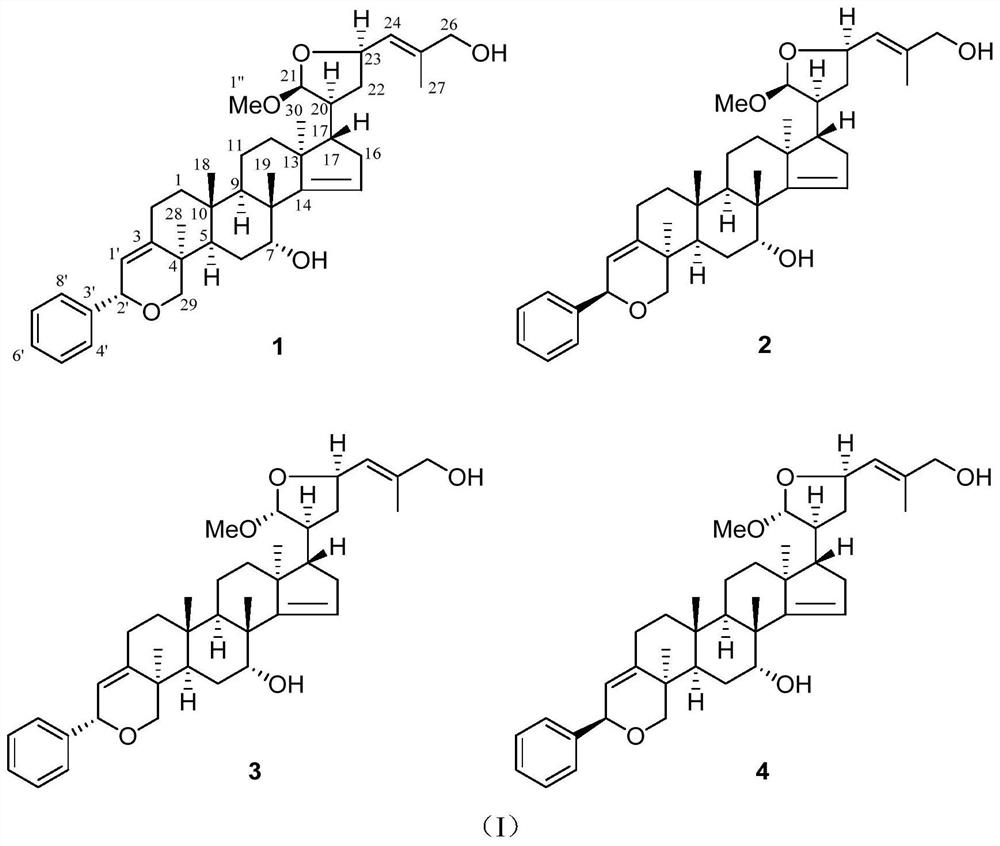
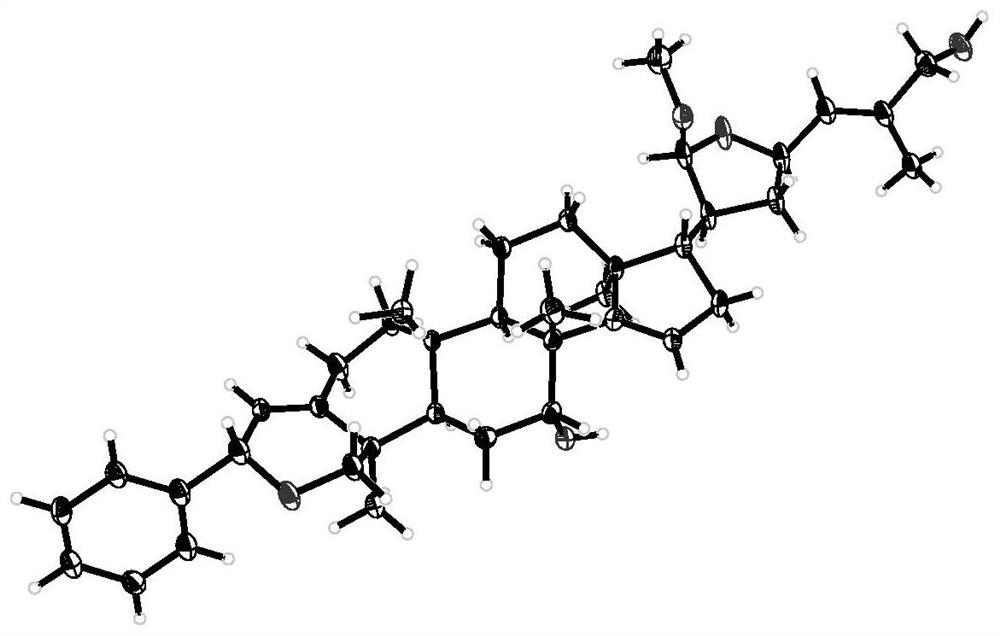
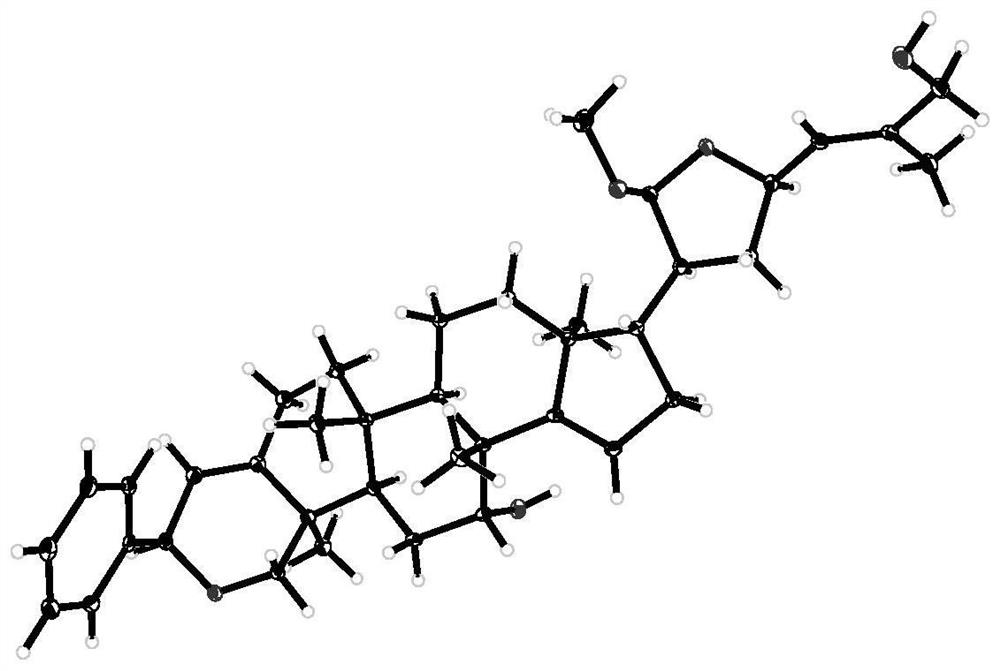
[0029] Preparation and evaluation of anticancer activity of compounds pacidusin A(1) and pacidusin C(3).
[0030] The preparation of compound pacidusin A (1) and pacidusin C (3): the air-dried leaves (30kg) of Indian gooseberry were pulverized, soaked in 95% ethanol water (100L), and refluxed and extracted 3 times (2h / time) at 60°C ). After filtration, the filtrate was concentrated under reduced pressure to remove the organic solvent to obtain 4.5 kg of crude extract. The crude extract was extracted with water and ethyl acetate, and the ethyl acetate part (2.0kg) was subjected to silica gel column chromatography, using petroleum ether: ethyl acetate (20:1,10:1,8:1,2:1,1 :1 and 0:1) solution for gradient elution to obtain 6 main parts (A-F), part B was subjected to MCI column chromatography, eluted with 90% methanol water to obtain 72g yellow gum, this part was continued to use silica gel Column chromatography, carry out gradient elution with petroleum ether: acetone (9:1-1:2...
Embodiment 2
[0037] Preparation and evaluation of anticancer activity of compounds pacidusin B(2) and pacidusin D(4).
[0038] Preparation of compounds pacidusinB(2) and pacidusinD(4): Air-dried leaves of Indian gooseberry (30kg) were pulverized, soaked in 95% ethanol water (100L), and refluxed at 60°C for 3 times (2h / time). After filtration, the filtrate was concentrated under reduced pressure to remove the organic solvent to obtain 4.5 kg of crude extract. The crude extract was extracted with water and ethyl acetate, and the ethyl acetate part (2.0kg) was subjected to silica gel column chromatography, using petroleum ether: ethyl acetate (20:1,10:1,8:1,2:1,1 :1 and 0:1) solution for gradient elution to obtain 6 main parts (A-F), part B was subjected to MCI column chromatography, eluted with 90% methanol water to obtain 72g yellow gum, this part was continued to use silica gel Column chromatography, carry out gradient elution with petroleum ether: acetone (9:1-1:2) solution, obtain 6 fra...
preparation Embodiment 1
[0056] According to the method of Examples 1 and 2, the air-dried leaves (30 kg) of Indian gooseberry were crushed, soaked in 95% ethanol water (100 L), and refluxed at 60° C. for 3 times (2 h / time). After filtration, the filtrate was concentrated under reduced pressure to remove the organic solvent to obtain 4.5 kg of crude extract. The crude extract was extracted with water and ethyl acetate, and the ethyl acetate part (2.0kg) was subjected to silica gel column chromatography, using petroleum ether: ethyl acetate (20:1,10:1,8:1,2:1,1 :1 and 0:1) solution for gradient elution to obtain 6 main parts (A-F), part B was subjected to MCI column chromatography, eluted with 90% methanol water to obtain 72g yellow gum, this part was continued to use silica gel Column chromatography, carry out gradient elution with petroleum ether: acetone (9:1-1:2) solution, obtain 6 fractions (B 1 -B 6 ). B 2 Compound 1-4 was partially obtained by using preparative liquid phase (85% methanol-wat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com