CRSPR-cas13a driven RNA rapid detection method based on double-enzyme signal amplification strategy
A detection method and enzyme signal technology, which is applied in the field of rapid RNA detection based on a dual-enzyme signal amplification strategy, can solve problems such as difficulty in reaching the detection range of nucleic acid sequence concentration, limited RNA content in biological fluids, and complex components in biological fluids. Sensitivity, fast separation speed, and simple instrument requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] The following will clearly and completely describe the technical solutions in the embodiments of the present invention with reference to the accompanying drawings in the embodiments of the present invention. Obviously, the described embodiments are only some, not all, embodiments of the present invention. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
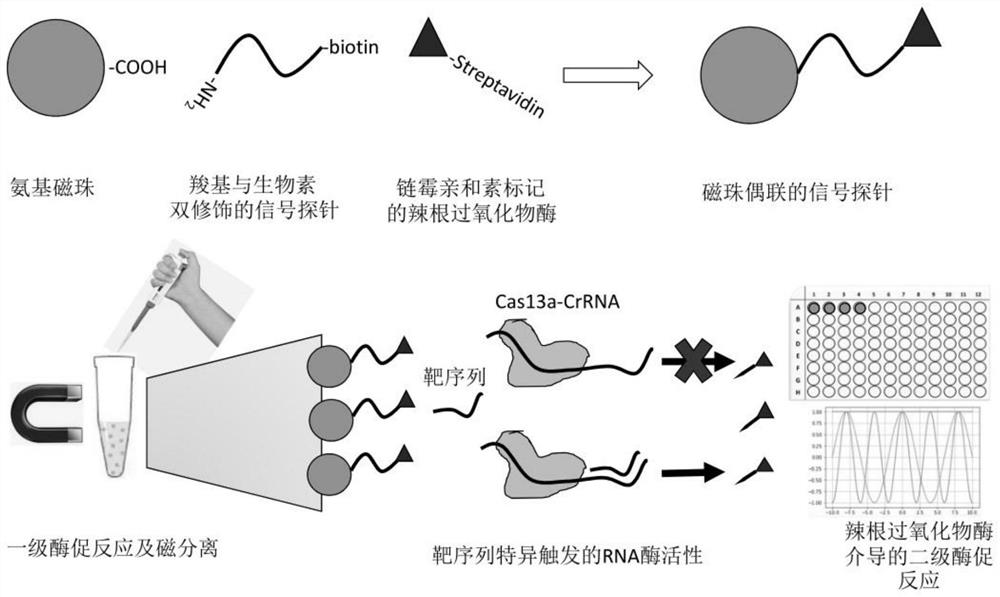
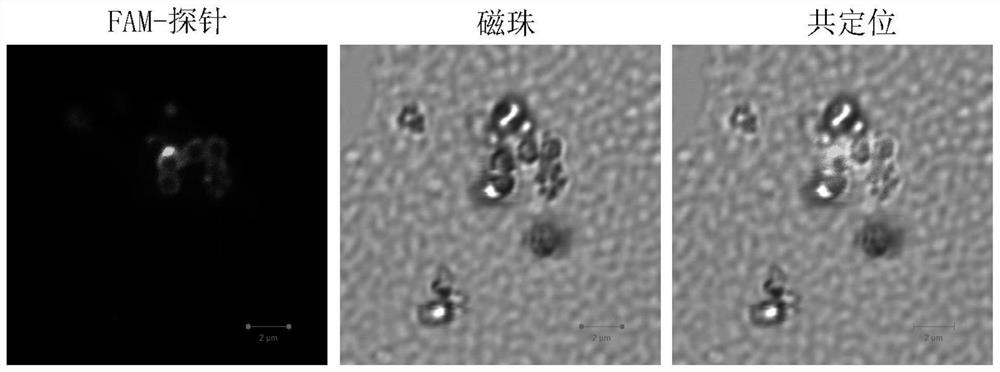
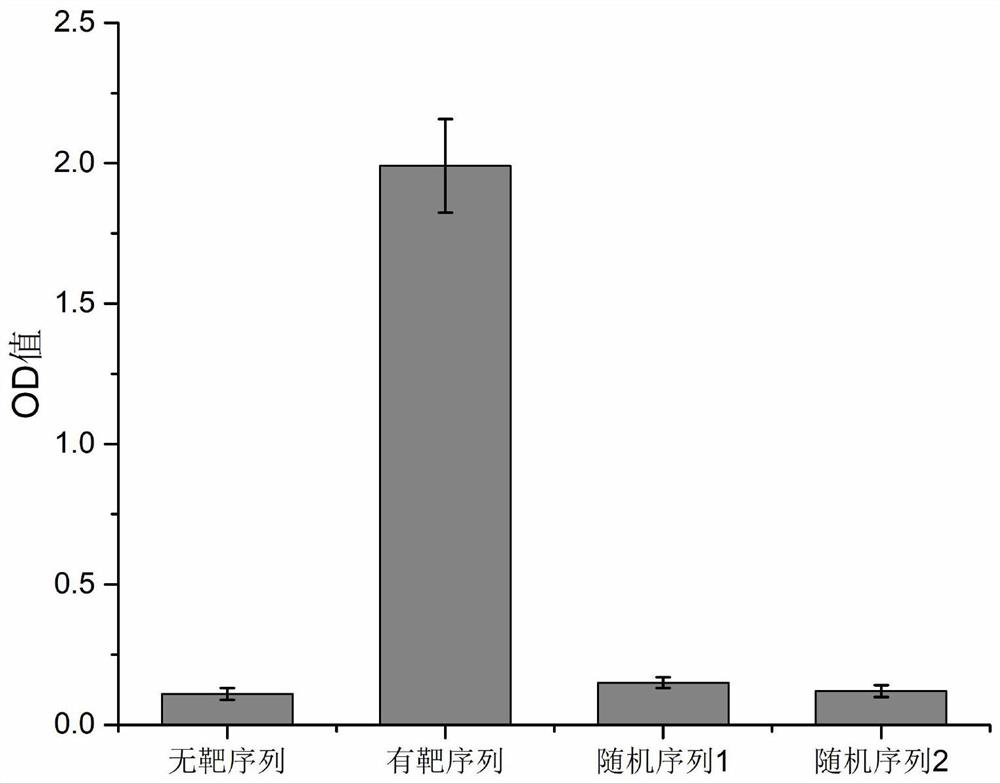
[0033] The invention provides a CRSPR-cas13a-driven RNA rapid detection method based on a dual-enzyme signal amplification strategy. The nucleic acid detection sequence is controllable, and a suitable guide sequence can be designed according to actual needs to control the recognized target sequence. It has high specificity and high sensitivity, and has good color rendering effect. In the case of qualitative analysis, the test result can be judged by naked eyes ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com