Small cell lung cancer therapeutic agent containing oligonucleotide
An oligonucleotide and nucleic acid technology, applied in the field of small cell lung cancer therapeutic drugs containing oligonucleotides, can solve the problems of inability to use by patients and high incidence of diarrhea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0449] (Example 1: Oligonucleotide Synthesis)
[0450] The oligonucleotides related to the present invention were synthesized by the methods described in Tetrahedron Letters 22, 1859-1862 (1981), International Publication No. 2011 / 052436 and the like.
[0451] Specifically, GeneDesign Co., Ltd. was requested to synthesize an oligonucleotide comprising 2',4'-BNA / LNA represented by the formula (a).
[0452]
[0453] (In the formula, Base is 5-methylcytosine, thymine, adenine or guanine.)
[0454] The oligonucleotide containing amide BNA (AmNA) represented by the formula (b) was synthesized by referring to the method described in International Publication No. 2011 / 052436.
[0455]
[0456] (In the formula, Base is 5-methylcytosine, thymine, adenine or guanine, and Me is methyl.)
[0457] An oligonucleotide of 15 to 19 bases (mer) comprising 2′,4′-BNA / LNA represented by formula (a) or amide BNA (AmNA) represented by formula (b) uses an automatic nucleic acid synthesizer (...
Embodiment 2
[0459] (Example 2: Design of antisense oligonucleotides)
[0460] Antisense oligonucleotides were designed to target the mRNA of human nSR100 (hnSR100) (GenBank: NM_194286.3 (SEQ ID NO: 1)).
[0461] In order to select the target region, reverse sequences (CG, GGA, GCA) of CG, TCC, and TGC that exhibit toxicity when antisense are removed. Then, based on the secondary structure prediction using mfold (mfold: http: / / unafold.rna.albany.edu / ?q=mfold), regions that are easily accessible to antisense oligonucleotides such as circular structures were selected. Next, using Blast (BLAST: https: / / blast.ncbi.nlm.nih.gov / Blast.cgi?PAGE_TYPE=BlastSearch), the nSR100 gene was centered on the region with high identity between human mRNA and mouse mRNA Selected so that the results obtained based on the evaluation in mice can be applied to humans. Thus, 22 candidate sequences were selected.
[0462] An oligonucleotide having a base sequence complementary to the candidate sequence selected a...
Embodiment 3
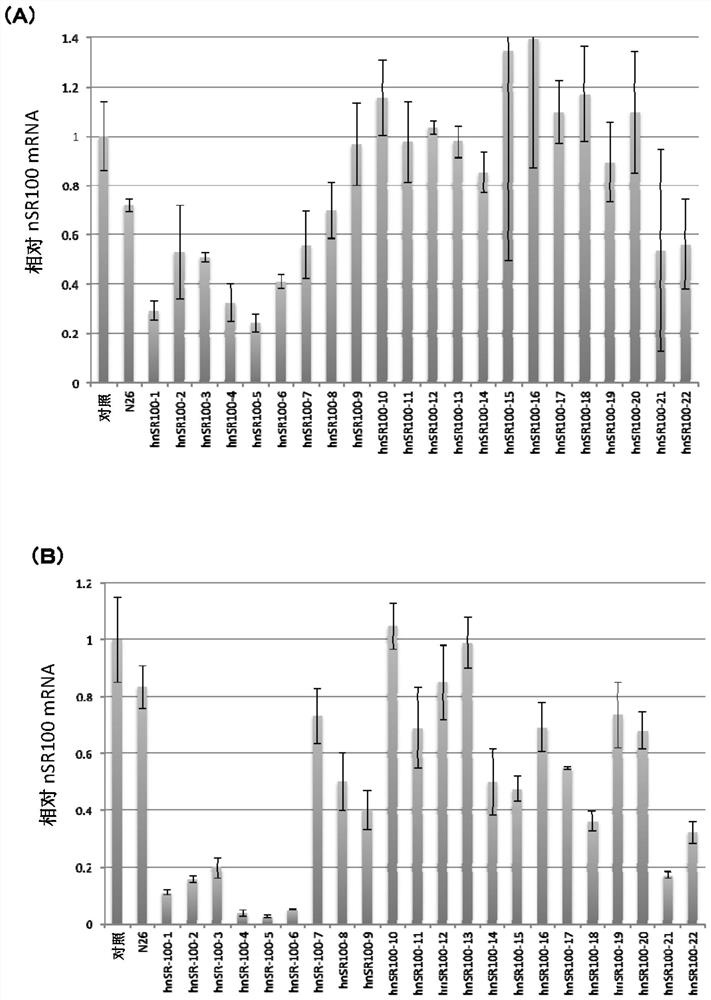
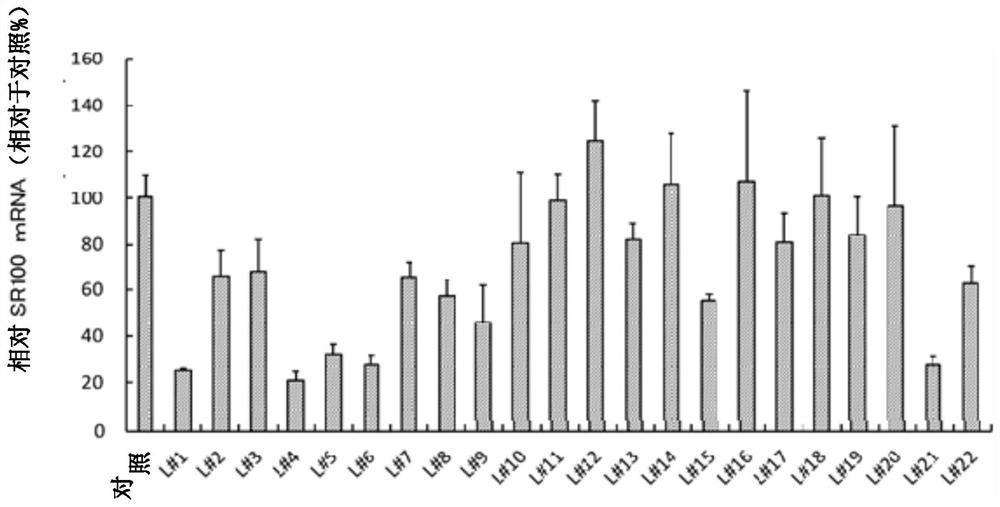
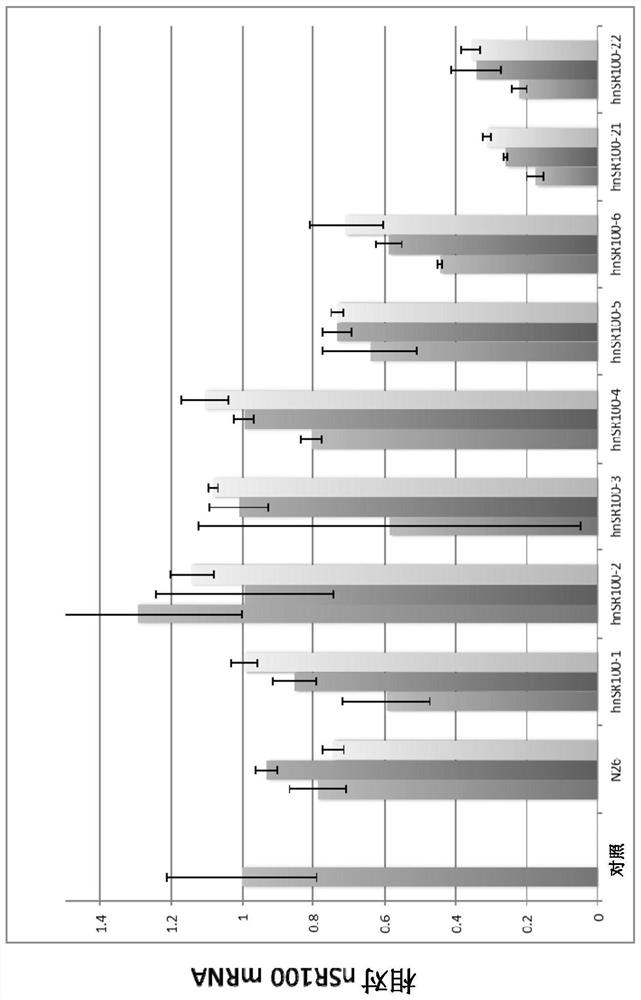
[0472] (Example 3: Inhibition of mRNA expression of nSR100 in vitro in human SCLC cells)
[0473] (3-1: Analysis of mRNA expression inhibition by various antisense oligonucleotides)
[0474] For the antisense oligonucleotides prepared in Example 2, mRNA expression suppression of nSR100 in human SCLC cells in vitro was investigated. As human SCLC cells, NCI-H82 cells (American Type Culture Collection: ATCC), STC-1 cells (ATCC) and NCI-N417 cells (ATCC) were used. Cells without oligonucleotide addition served as controls. In addition, as a comparison, an N26 oligonucleotide was used (base sequence: 5'-TGAacaaaataaTAc-3': in this example, bases in capital letters are 2', 4'-BNA / LNA, and bases in lowercase letters The base is DNA, S-oligonucleotide: sequence number 100).
[0475] Each antisense oligonucleotide was passed through the CEM method (using "Ca 2+ "enrichment for medium" method of calcium ion-rich medium: Nucleic Acids Research, 2015, Vol.43, e128) into human SCLC ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com