Liquid preparation of CD47 monoclonal antibody and preparation method of liquid preparation
A monoclonal antibody, liquid preparation technology, applied in the direction of antibodies, medical components of antibodies, medical preparations of non-active components, etc., can solve problems such as stability effects, and achieve the effect of good stability and low quantity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
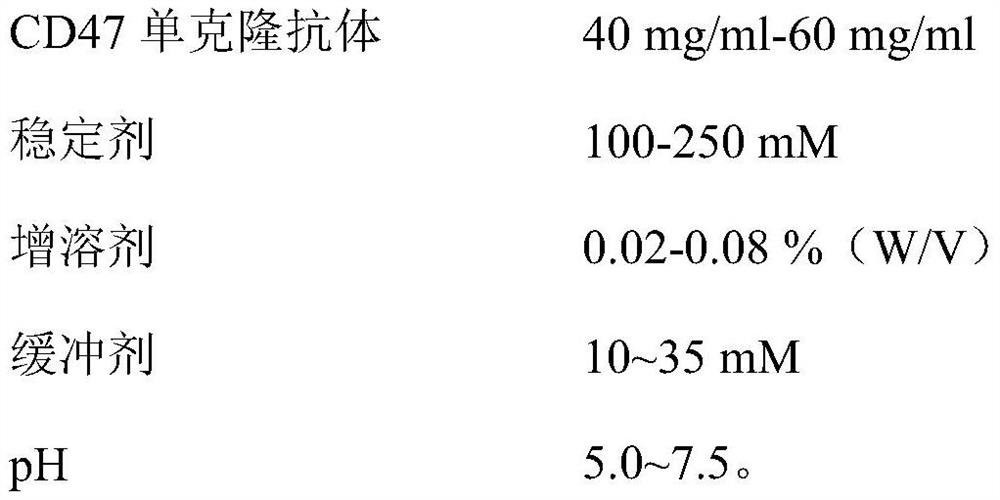
[0027] (1) Formulation:
[0028]
[0029] (2) Preparation method
[0030] Weigh the prescribed amount of buffer, add water for injection to dissolve, adjust the pH to 5.5, use the buffer system obtained above to concentrate the CD47 antibody protein stock solution by ultrafiltration, replace the original buffer system of the CD47 antibody protein, and then add the prescribed amount of chitosan and Egg yolk lecithin, stirred evenly, then adjusted the CD47 protein concentration to 50 mg / ml with a buffer system, sterile filtered through a 0.22 μm filter membrane, and then filled.
Embodiment 2
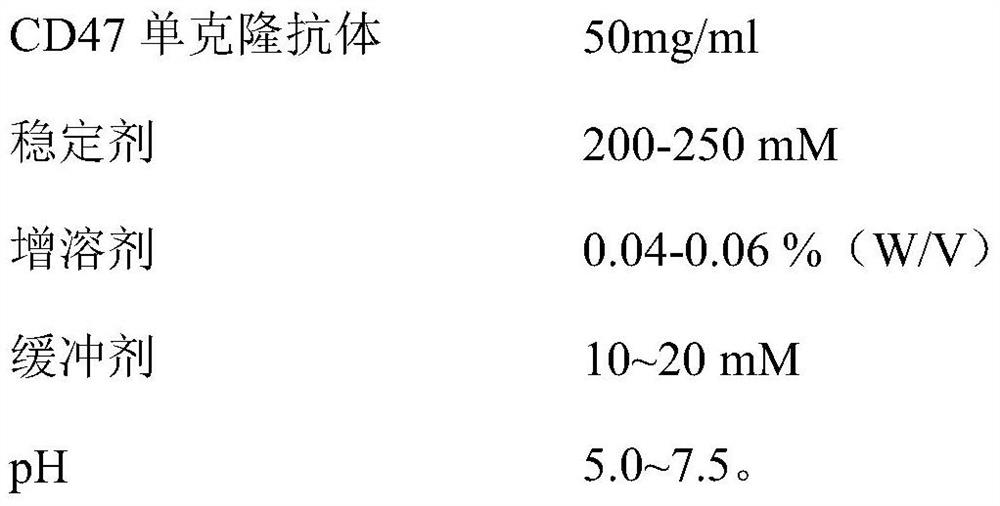
[0032] (1) Formulation:
[0033]
[0034] (2) Preparation method
[0035] Weigh the prescribed amount of buffer, add water for injection to dissolve, adjust the pH to 6.0, use the buffer system obtained above to concentrate the CD47 antibody protein stock solution by ultrafiltration, replace the original buffer system of the CD47 antibody protein, and then add the prescribed amount of trehalose and egg yolk Lecithin, stir evenly, then adjust the CD47 protein concentration to 50 mg / ml with a buffer system, filter aseptically through a 0.22 μm filter membrane, and then fill it.
Embodiment 3
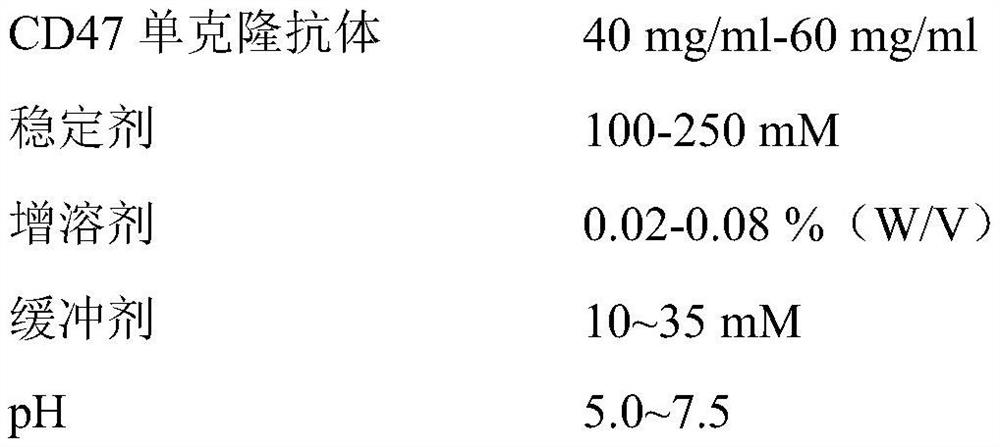
[0037] (1) Formulation:
[0038]
[0039] (2) Preparation method
[0040] Weigh the prescribed amount of buffer, add water for injection to dissolve, adjust the pH to 5.0, use the buffer system obtained above to concentrate the CD47 antibody protein stock solution by ultrafiltration, replace the original buffer system of the CD47 antibody protein, and then add the prescribed amount of sucrose and asparagus Amide, stir evenly, then adjust the CD47 protein concentration to 60mg / ml with a buffer system, perform sterile filtration through a 0.22μm filter membrane, and then fill it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com