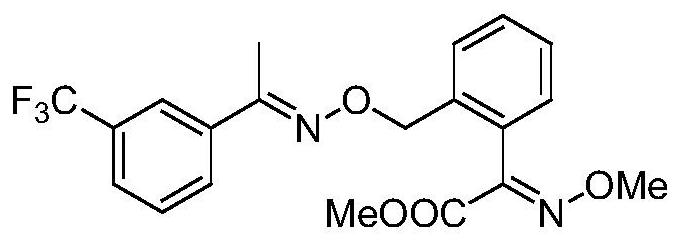
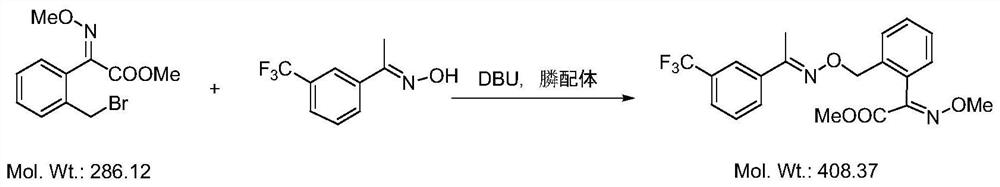
Green synthesis method for high-yield preparation of trifloxystrobin
A technology for green synthesis and trifloxystrobin, which is applied in oxime preparation, organic chemistry methods, chemical instruments and methods, etc., can solve the problems of low overall yield, large amount of inorganic waste salts, cumbersome work sections, etc., and improve the reaction rate. , The effect of reducing the amount of waste salt and improving the final yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
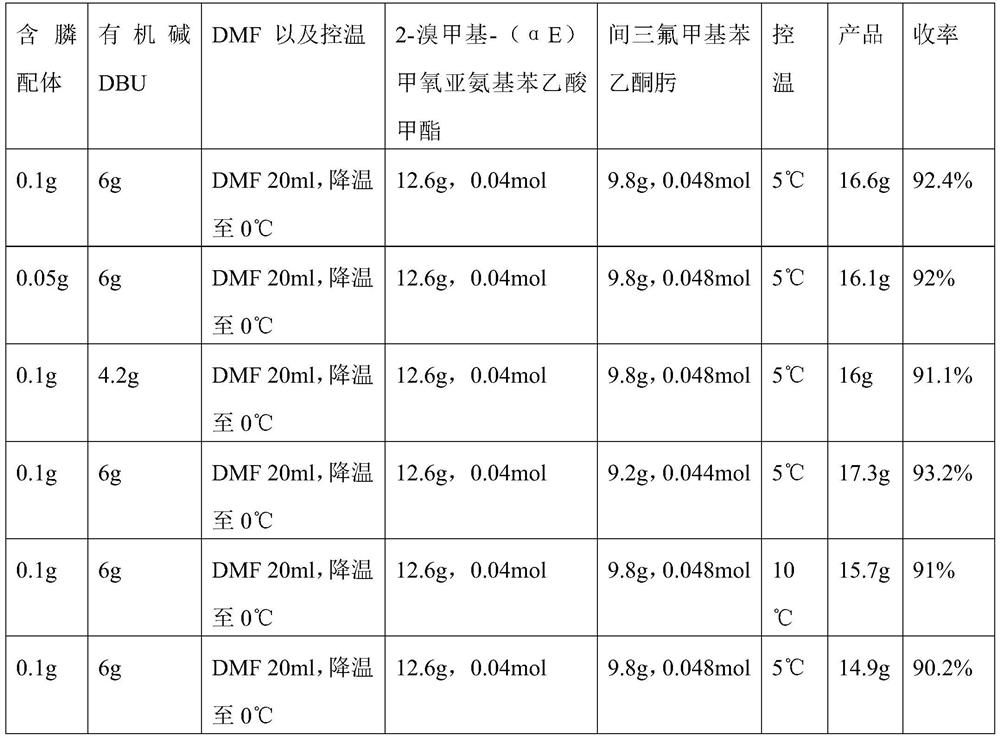
[0027] Add 0.1 g of phosphine-containing ligand, 6 g of organic base DBU (1,8-diazabicycloundec-7-ene) and 20 ml of DMF into a 250 mL three-necked flask, stir to dissolve, and cool down to 0°C. After cooling, a mixed solution of 2-bromomethyl-(αE)methoxyiminophenylacetic acid methyl ester (12.6g, 0.04mol) and m-trifluoromethylacetophenone oxime (9.8g, 0.048mol) was added dropwise (contains DMF40ml), temperature controlled at 5°C and added dropwise. After the dropwise addition, keep warm for 10 minutes, then raise the temperature to 15°C and keep warm for 2 hours. After the completion of the detection reaction, cool down to room temperature and slowly add 160ml of water to it, continue to stir, a white solid precipitates out, filter and drain to obtain 17.4g of crude product. The crude product was dissolved in 60ml of ethanol, recrystallized to obtain 16.6g of pure white solid, the purity determined by liquid chromatography was 96.84%, and the yield was 92.4%.
[0028] The NM...
Embodiment 2
[0031] Add 0.05 g of phosphine-containing ligand, 6 g of organic base DBU (1,8-diazabicycloundec-7-ene) and 20 ml of DMF into a 250 mL three-necked flask, stir to dissolve, and cool down to 0°C. After cooling, a mixed solution of 2-bromomethyl-(αE)methoxyiminophenylacetic acid methyl ester (12.6g, 0.04mol) and m-trifluoromethylacetophenone oxime (9.8g, 0.048mol) was added dropwise (contains DMF40ml), temperature controlled at 5°C and added dropwise. After the dropwise addition, keep warm for 10 minutes, then raise the temperature to 15°C and keep warm for 2 hours. After the completion of the detection reaction, cool down to room temperature and slowly add 160ml of water to it, continue to stir, a white solid precipitates out, filter and drain to obtain 17g of crude product. The crude product was dissolved in 60 ml of ethanol, and recrystallized to obtain 16.1 g of pure white solid. The purity determined by liquid chromatography was 96.2%, and the yield was 92%.
[0032] The ...
Embodiment 3
[0035] Add 0.1 g of phosphine-containing ligand, 4.2 g of organic base DBU (1,8-diazabicycloundec-7-ene) and 20 ml of DMF into a 250 mL three-necked flask, stir to dissolve, and cool down to 0°C. After cooling, a mixed solution of 2-bromomethyl-(αE)methoxyiminophenylacetic acid methyl ester (12.6g, 0.04mol) and m-trifluoromethylacetophenone oxime (9.8g, 0.048mol) was added dropwise (contains DMF40ml), temperature controlled at 5°C and added dropwise. After the dropwise addition, keep warm for 10 minutes, then raise the temperature to 15°C and keep warm for 2 hours. After the completion of the detection reaction, cool down to room temperature and slowly add 160ml of water to it, continue to stir and a white solid precipitates out, filter and drain to obtain 16.8g of crude product. The crude product was dissolved in 60ml of ethanol, and recrystallized to obtain 16g of pure white solid. The purity determined by liquid chromatography was 96.2%, and the yield was 91.1%.
[0036] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com