Method for determining genotoxic substance 1, 2-dibromoethane of loratadine ring compound
A technology of loratadine and dibromoethane, which is applied in the field of analytical chemistry, can solve the problems of incomplete removal of genotoxic substances and affect the safety of medication, and achieve the effect of ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Instruments and Conditions
[0039] Chromatograph: Agilent 6890N gas chromatograph;
[0040] Detector: electron capture detector;
[0041] Chromatographic column: 6% cyanopropyl-94% dimethylpolysiloxane capillary column;
[0042] Injection port temperature: 150°C;
[0043] Detector temperature: 250°C;
[0044] Carrier gas (nitrogen) flow rate: 3.0mL / min;
[0045] Split ratio: 10:1;
[0046] Injection volume: 1μL
[0047] Oven heating program:
[0048] Heating rate (℃ / min) temperature (°C) Hold time (min) / 100 0 8 120 5
[0049] Experimental procedure
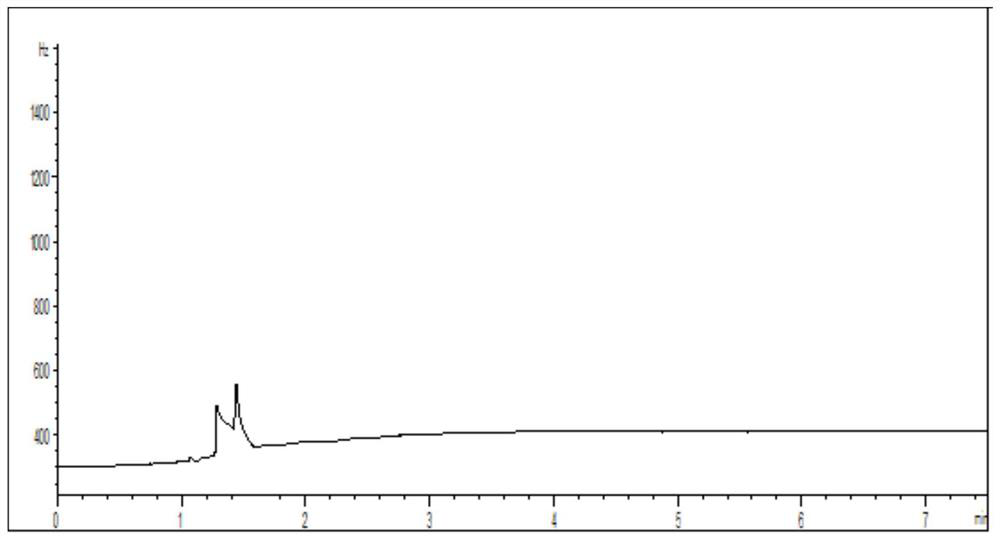
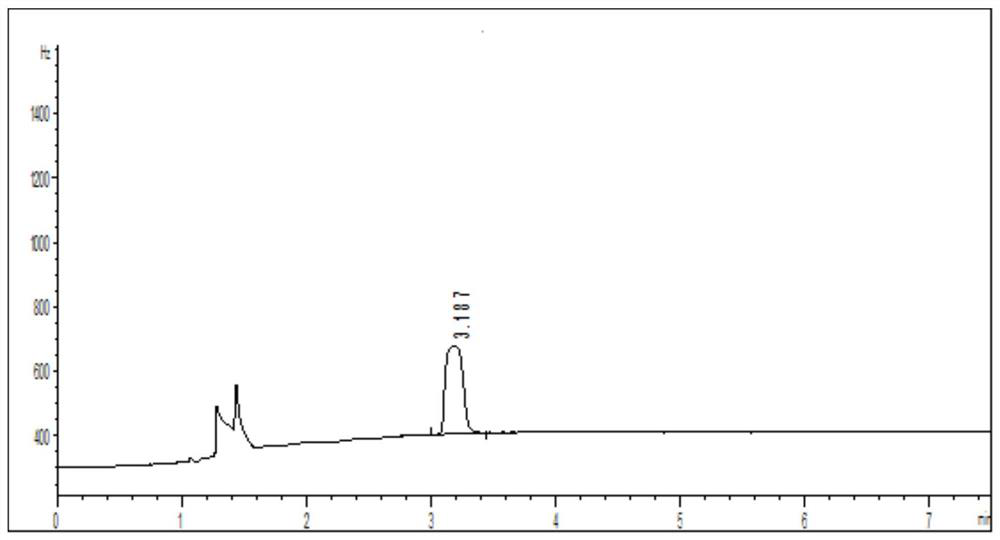
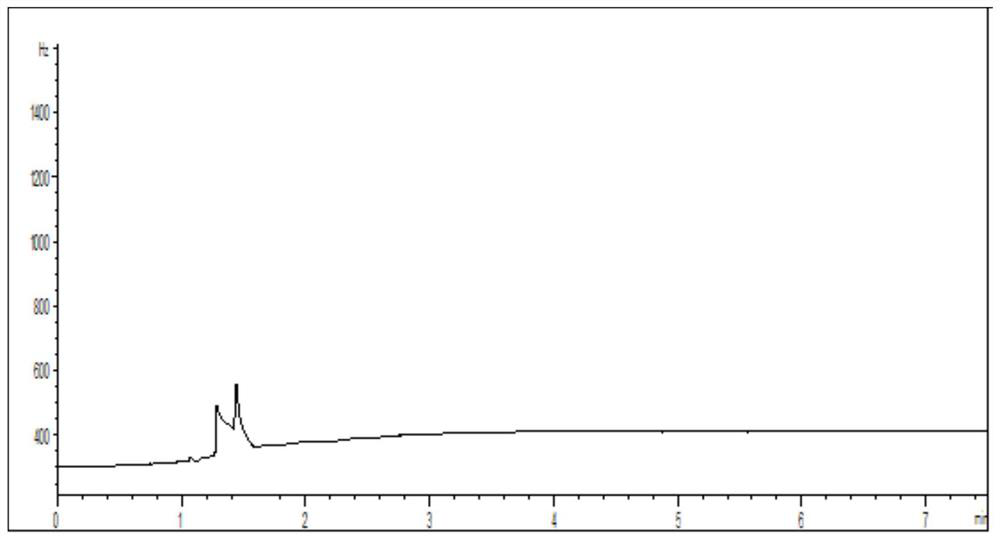
[0050] Get 100mg of loratadine cyclic compound, dissolve it with solvent, be mixed with the need testing solution that every 1mL contains loratadine cyclic compound 100mg; Another get 1,2-dibromoethane appropriate amount, dissolve with solvent Dissolve it, and make it into a reference substance solution containing 0.0005 mg of 1,2-dibromoethane per 1 mL; take another 100 mg of lorata...
Embodiment 2
[0052] Instruments and Conditions
[0053] Chromatograph: Agilent 6890N gas chromatograph;
[0054] Detector: electron capture detector;
[0055] Chromatographic column: 6% cyanopropyl-94% dimethylpolysiloxane capillary column;
[0056] Inlet temperature: 170°C;
[0057] Detector temperature: 250°C;
[0058] Carrier gas (nitrogen) flow rate: 2mL / min;
[0059] Split ratio: 10:1;
[0060] Injection volume: 1μL
[0061] Oven heating program:
[0062] Heating rate (℃ / min) temperature (°C) Hold time (min) / 100 0 8 120 5
[0063] Experimental procedure
[0064] Take an appropriate amount of 1,2-dibromoethane, dissolve it in a solvent, and prepare a reference solution containing 0.0005 mg of 1,2-dibromoethane per 1 mL; take another 100 mg of loratadine ring compound, add 1.0 mL reference substance solution, prepared into a system suitability solution containing 100 mg of loratadine ring compound per 1 mL. Take the system suitability solution, ana...
Embodiment 3
[0066] Instruments and Conditions
[0067] Chromatograph: Agilent 6890N gas chromatograph;
[0068] Detector: electron capture detector;
[0069] Chromatographic column: 6% cyanopropyl-94% dimethylpolysiloxane capillary column;
[0070] Injection port temperature: 150°C;
[0071] Detector temperature: 250°C;
[0072] Carrier gas (nitrogen) flow rate: 3.0mL / min;
[0073] Split ratio: 20:1;
[0074] Injection volume: 1μL
[0075] Oven heating program:
[0076] Heating rate (℃ / min) temperature (°C) Hold time (min) / 100 0 8 120 5
[0077] Experimental procedure
[0078] Take an appropriate amount of 1,2-dibromoethane, dissolve it in a solvent, and prepare a reference solution containing 0.0005 mg of 1,2-dibromoethane per 1 mL; take another 100 mg of loratadine ring compound, add 1.0 mL reference substance solution, prepared into a system suitability solution containing 100 mg of loratadine ring compound per 1 mL. Take the system suitability ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com