Cd80 extracellular domain fc fusion protein dosing regimens
A technology of extracellular domain and fusion protein, which is applied in the field of administration of CD80 extracellular domain Fc fusion protein, which can solve the problems of response, relapse, and non-response of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
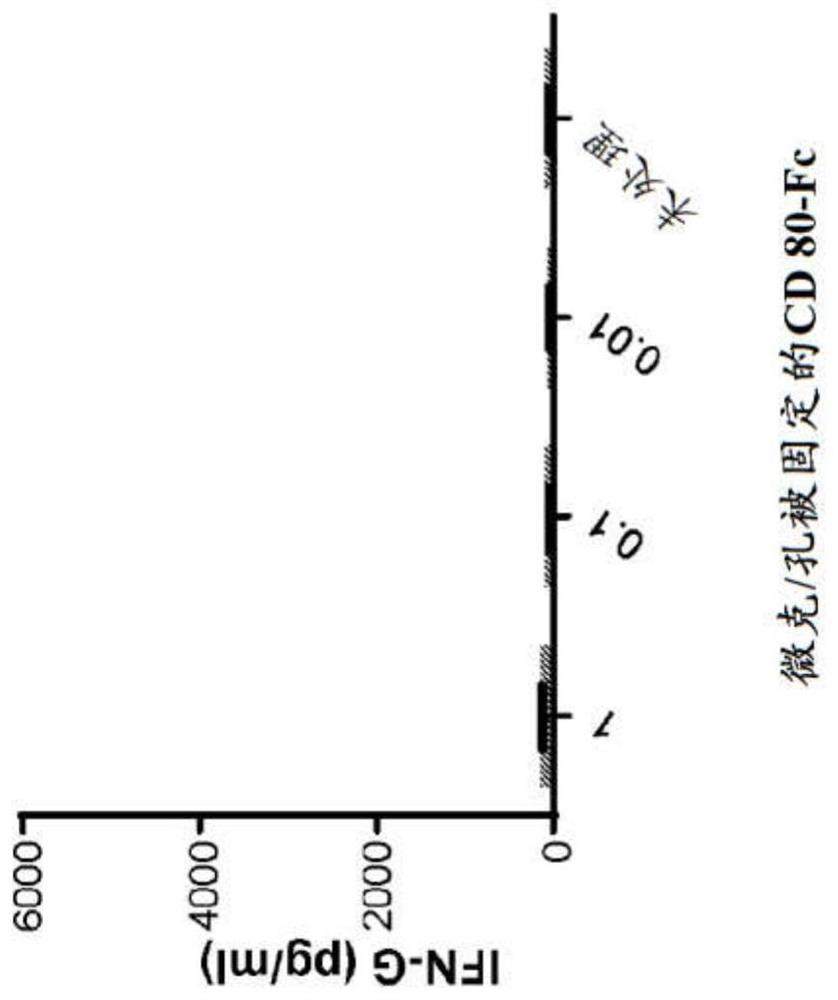
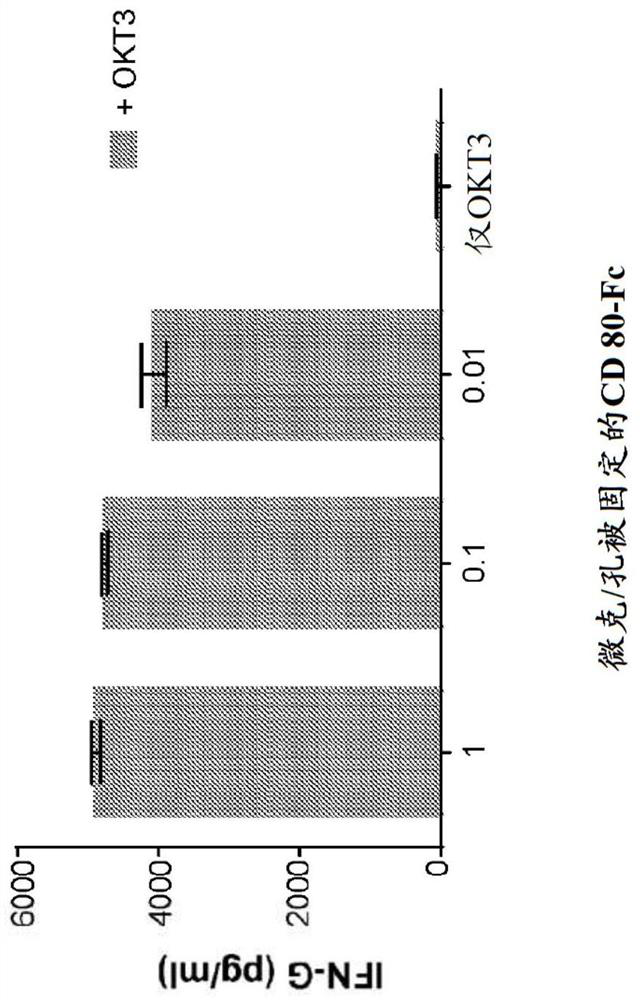
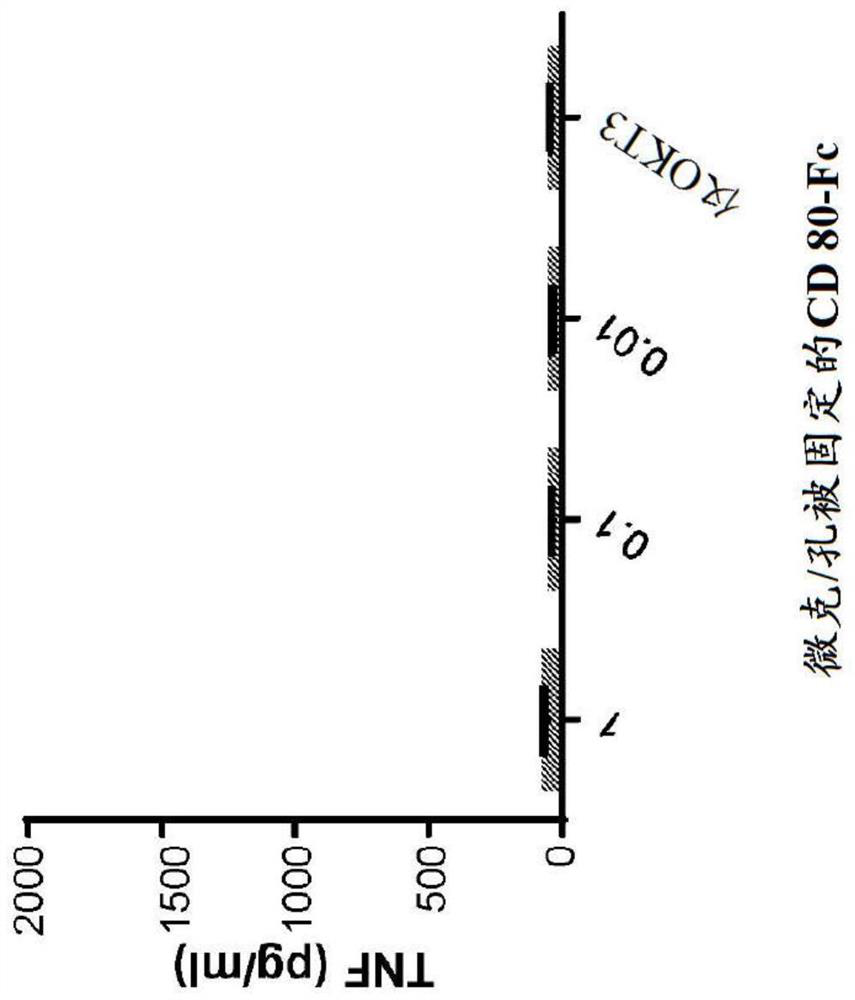
[0085] Example 1: Cytokine Release of CD80 ECD-Fc Fusion Molecules
[0086] method
[0087] protein treatment
[0088] In the T containing RPMI 1640, 100IU penicillin (Penicillin) / 100ug / ml streptomycin (Streptomycin), 2mM L-glutamine, 100nM non-essential amino acids, 55uM 2-mercaptoethanol and 10% ultra-low-IgG fetal bovine serum Human CD80 ECD IgGl Fc fusion protein ("CD80-Fc") was bound to magnetic protein-A beads (Life Technologies) in cell proliferation medium. Binding reactions were performed at a bead concentration of three million beads per milliliter in a volume of 100 μl per well in 96-well flat bottom tissue culture plates. CD80-Fc was bound to beads in a range of concentrations: 10 μg / ml, 1 μg / ml, 0.1 μg / ml. Another set of binding reactions was also performed with the addition of 3 ng / ml OKT3-scFv. The proteins were allowed to bind for 1 hour at room temperature on a rocking platform, after which 100 μl of 20 μg / ml (final concentration 10 μg / ml) IgG1 Fc-free (...
Embodiment 2
[0097] Example 2: Effects of CD80ECD-Fc fusion molecules on CT26 tumors in vivo with different sialic acid (SA) contents in the Fc domain
[0098] An in vivo study was performed in CT26 tumors to analyze the effect of three different lots of CD80 ECD with different sialic acid (SA) content fused with wild-type human IgGl Fc. Specifically, Lot E of CD80 ECD-Fc contained 20 mol SA / mol protein, Lot D contained 15 mol SA / mol protein, and Lot A contained 5 mol SA / mol protein.
[0099] Seven-week-old female BALB / c mice were purchased from Charles River Laboratories (Hollister, CA) and acclimatized for one week prior to the start of the study. The murine colorectal cancer cell line CT26 was treated at 1.0×10 6 Cells / 200 μl / mouse were implanted subcutaneously in the right lumbar fossa of the mice. Prior to inoculation, cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, and passaged up to three times. cells in a...
Embodiment 3
[0105] Example 3: Effect of murine CD80 ECD-murine Fc fusion molecule on tumor growth in three different syngeneic tumor models
[0106] In vivo studies were performed using a mouse surrogate comprising the extracellular domain (ECD) of murine CD80 linked to the Fc domain of mouse IgG2a wild type (murine CD80 ECD-Fc). The effect of murine CD80ECD-Fc was compared to that of anti-CTLA4 antibody clone 9D9 (IgG2b) in three different syngeneic tumor models: CT26 colon carcinoma, MC38 colon carcinoma and B16 melanoma models.
[0107] CT26 tumor model
[0108] Seven-week-old female BALB / c mice were purchased from Charles River Laboratories (Hollister, CA) and acclimatized for one week prior to the start of the study. The murine colorectal cancer cell line CT26 was treated at 1.0×10 6 Cells / 200 μl / mouse were implanted subcutaneously in the right lumbar fossa of the mice. Prior to inoculation, cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com