Mesenchymal stem cell preparation and preparation method and application thereof
A technology of stem cells and preparations, applied in the field of medicine, can solve the problem that bone repair materials cannot take into account the porosity and mechanical strength at the same time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] This embodiment provides a preparation of mesenchymal stem cells, including mesenchymal stem cells, where the mesenchymal stem cells are pretreated with immunosuppressive molecules.
[0047] The immunosuppressive molecule described in this embodiment is selected from prostaglandin E2.
[0048] The mesenchymal stem cell preparation described in this embodiment also includes at least one of pharmaceutically acceptable adjuvants, carriers and additives.
[0049]The process of pretreatment of the mesenchymal stem cells described in this embodiment by immunosuppressive molecules includes: providing mesenchymal stem cells, carrying out conventional culture of the mesenchymal stem cells using the first medium, and then using the second medium for the second medium. Cultivation; wherein, the first medium is a conventional medium for mesenchymal stem cells, and the second medium includes immunosuppressive molecules.
[0050] In the second culture medium, the working concentrati...
Embodiment 2
[0057] Example 2: Isolation of human umbilical cord mesenchymal stem cells
[0058] Discard the excess umbilical cord preservation solution in the obtained fresh umbilical cord, pour 75% alcohol for disinfection for three minutes, take out the umbilical cord, put it in physiological saline containing double antibodies and wash it repeatedly to remove the residual alcohol on the surface. Cut off the ligated part and discard it. Divide the remaining umbilical cord evenly into small pieces for cleaning, wash the residual blood repeatedly, remove the umbilical vein and umbilical artery, peel off the Wharton glue, and cut it to 1mm 3 The size of the organization block. According to the amount of tissue pieces, add an appropriate amount of conventional medium, mix evenly, spread it on a 15cm petri dish, and place it at 37°C, 5% CO 2 And cultivate in a humidity-saturated incubator for 24 hours, and add 8ml of culture solution to each culture dish. Replenish the fluid on the 5th day...
Embodiment 3
[0059] Embodiment 3: Preparation of human umbilical cord mesenchymal stem cell preparation
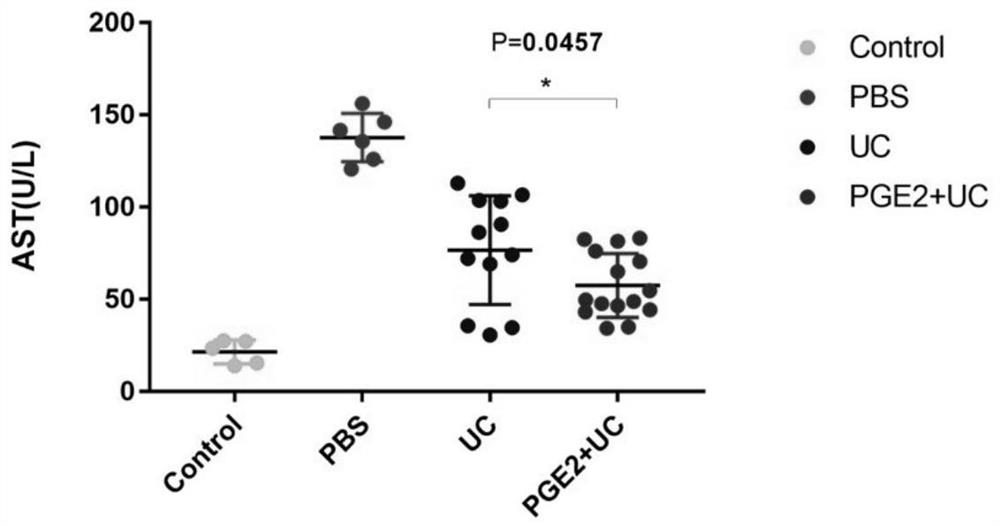
[0060] The first-generation umbilical cord mesenchymal stem cells with good growth status were selected, cultured in α-MEM medium containing 10% fetal bovine serum, continuously passaged to the third generation, and digested when the cell fusion rate reached 85%-90%. Count, at 8000 / cm 2 Inoculated into culture flasks, a part of the cells were routinely cultured for the UC treatment group. Part of the cells were replaced with the second medium containing prostaglandin E2 (PGE2) to continue culturing, which was used in the PGE2-UC treatment group. Culture to the fifth passage, digest and count when the cell fusion rate reaches 85%-90%. Finally, resuspend with normal saline, add an appropriate amount of heparin, and then carry out the next treatment experiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com