Method for synthesizing tauroursodeoxycholic acid under catalysis of boric acid ester
A technology of tauroursodeoxycholic acid and ursodeoxycholic acid is applied in the field of synthesizing tauroursodeoxycholic acid under the catalysis of borate ester, and can solve the problems of unsuitable industrial production and high cost of synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
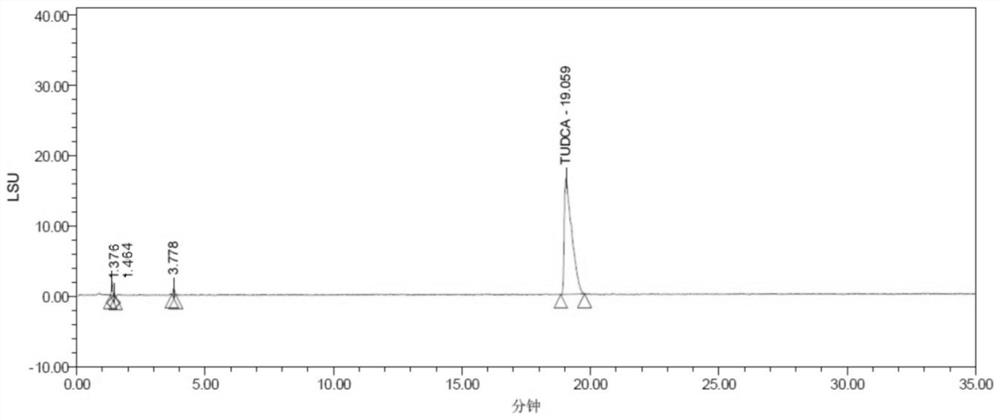
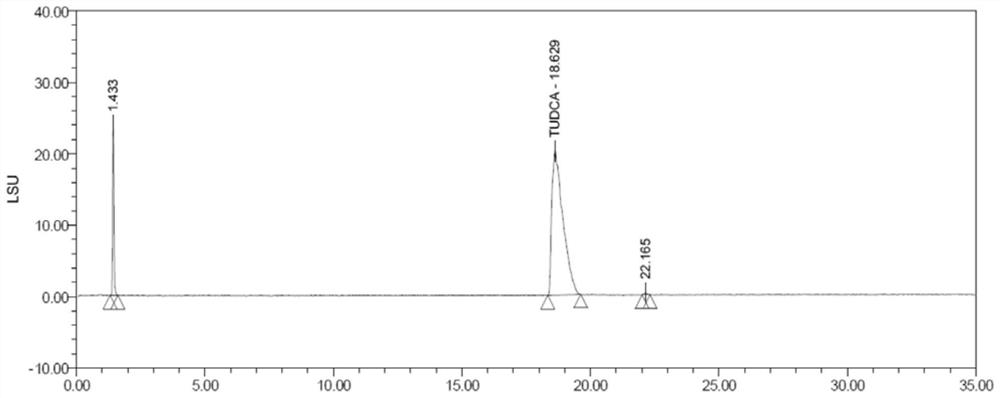
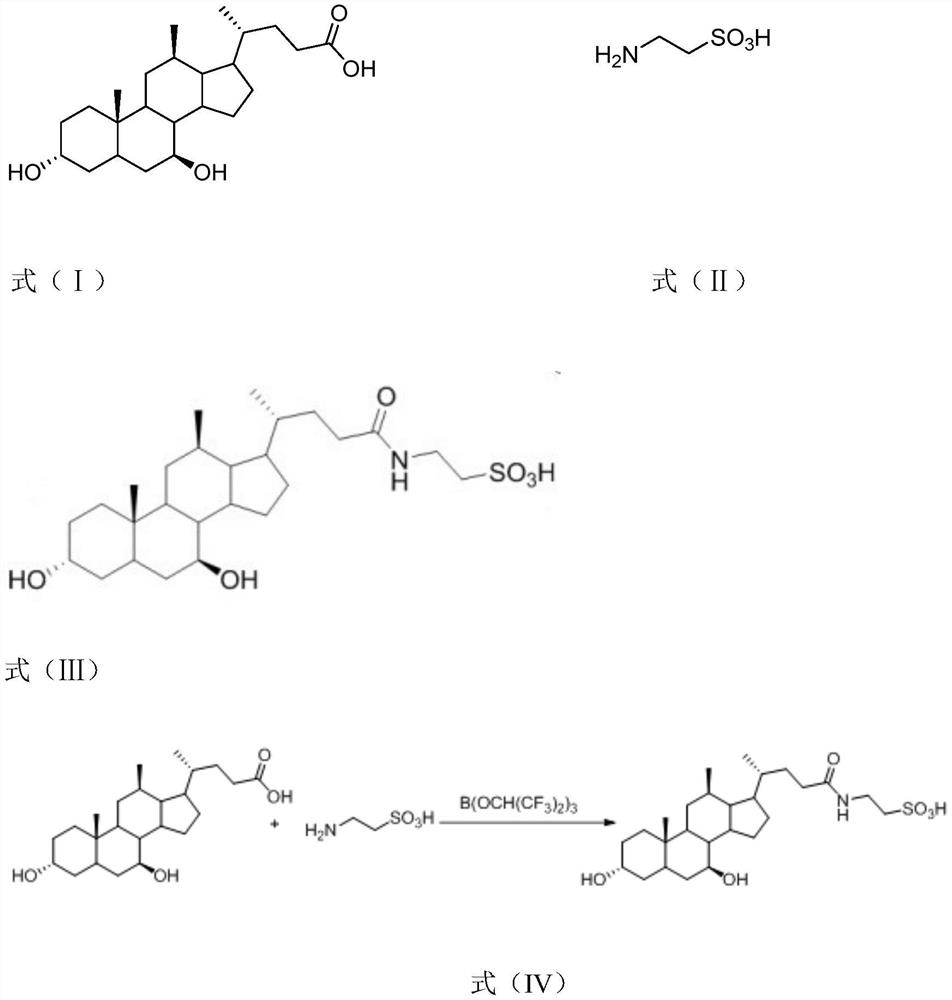
[0029] Ursodeoxycholic acid (cas: 128-13-2), taurine (cas: 07-35-7), and tauroursodeoxycholic acid (cas: 14605-22-2) are shown in formula (I) -shown in formula (III), the molecular formula of borate is: B(OCH(CF 3 ) 2 ) 3 , The synthesis process of tauroursodeoxycholic acid in this embodiment is shown in formula (IV).
[0030]
[0031] The method for synthesizing tauroursodeoxycholic acid under borate catalysis, comprises the steps:
[0032] S1: Add 1 mol of ursodeoxycholic acid to an organic solvent, place it on a magnetic stirrer, and stir evenly at a speed of 180 rpm. The organic solvent in this example is dichloromethane, ursodeoxycholic acid and organic The solvent addition ratio is 1g: 4ml;
[0033] S2: Add 1 mol of taurine and 1.5 mol of borate to the above-mentioned dissolved reaction system, after the addition is completed, react for 6 hours under reflux to obtain tauroursodeoxycholic acid;
[0034] S3: After the reaction, extract, wash, acidify, filter and co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com