Freeze-drying process of vancomycin hydrochloride
A vancomycin hydrochloride and freeze-drying technology, applied in the field of medicine, can solve the problems of easy crystallization or delamination of freeze-dried products, achieve easy industrial mass production, reduce high requirements on equipment and operations, and achieve the effect of appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
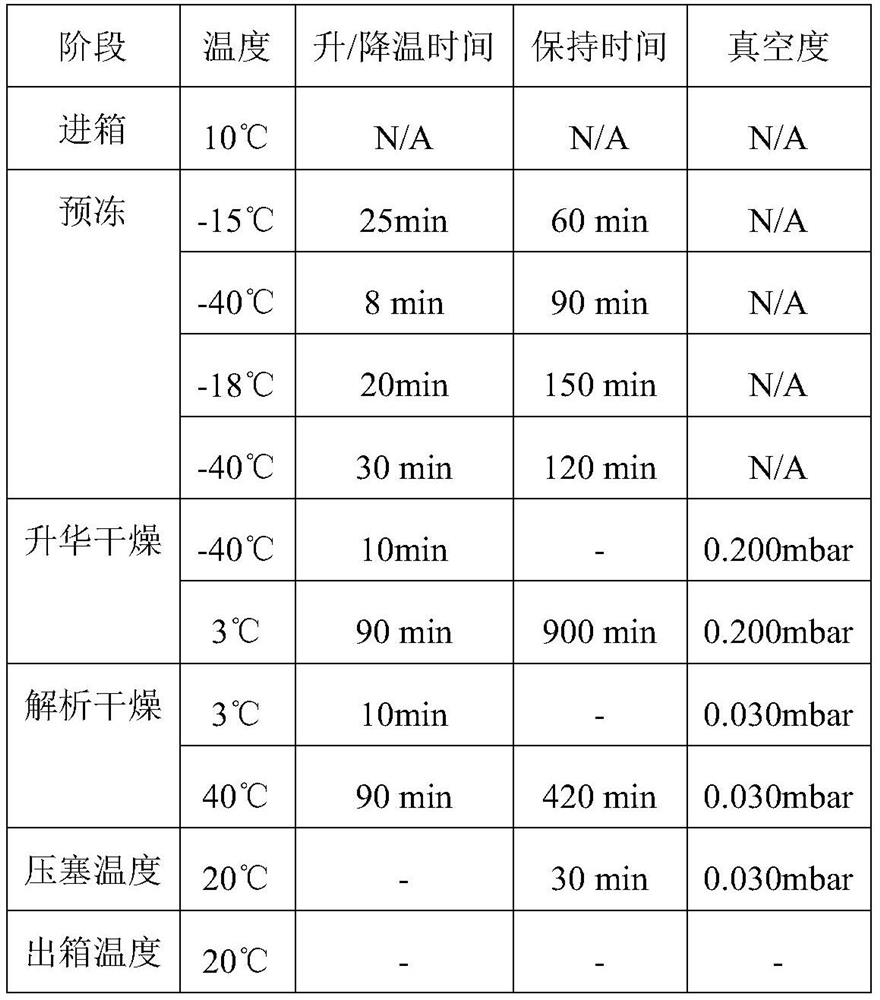
[0034] The freeze-drying process is shown in Table 1, and the state of the obtained freeze-dried powder is shown in figure 1 As shown, it can be seen that the obtained lyophilized powder is in a uniform loose amorphous state.
[0035] Table 1
[0036]
[0037] The composition of vancomycin hydrochloride stock solution is shown in Table 2.
[0038] Table 2
[0039]
[0040]
Embodiment 2
[0042] The freeze-drying process is shown in Table 3.
[0043] table 3
[0044]
[0045] The composition of the vancomycin hydrochloride stock solution is consistent with that of Example 1.
Embodiment 3
[0047] The freeze-drying process is shown in Table 4.
[0048] Table 4
[0049]
[0050]
[0051] The composition of the vancomycin hydrochloride stock solution is consistent with that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com