Application of liquid sodium methoxide in synthesis of alpha-acetyl-gamma-butyrolactone and synthesis method of alpha-acetyl-gamma-butyrolactone
A synthetic method and technology of butyrolactone, applied in the direction of organic chemistry, can solve the problems of high fire risk, large body injury, easy moisture absorption and spontaneous combustion of solid sodium methoxide, etc., and achieve the effect of reducing the risk of feeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] According to the second aspect of the present invention, a kind of synthetic method of α-acetyl-γ-butyrolactone is provided, comprising the following steps:
[0056] In the presence of liquid sodium methoxide, catalyzing the acylation reaction of acetate compounds and γ-butyrolactone to synthesize α-acetyl-γ-butyrolactone.
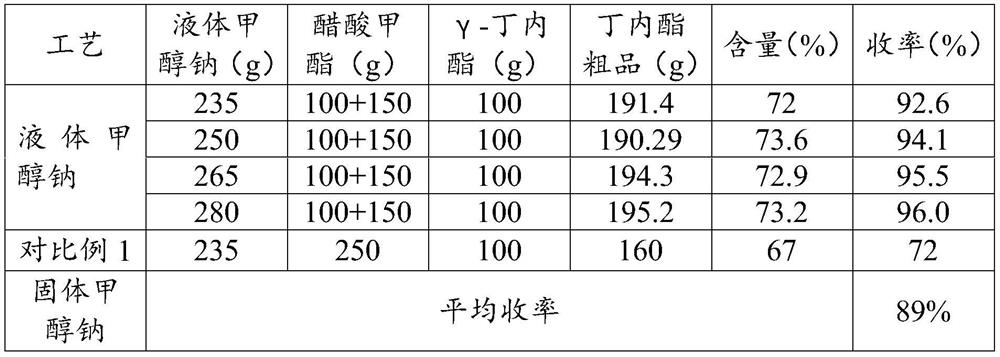
[0057] The present invention uses liquid sodium methoxide instead of solid sodium methoxide for acylation synthesis, realizes liquefaction and sealing of feeding, and reduces the risk of on-site feeding. Moreover, the synthetic yield of α-acetyl-γ-butyrolactone can be increased from 89% to over 96%.
[0058] Acetate compounds include, but are not limited to, methyl acetate (methyl acetate), ethyl acetate, and the like.
[0059] Specifically, the synthetic method of α-acetyl-γ-butyrolactone comprises the following steps:
[0060] (a) Acetate compound and gamma-butyrolactone carry out pre-acylation reaction;
[0061] Preferably, the mass ratio of a...
Embodiment 1
[0086] A preparation method of α-acetyl-γ-butyrolactone, comprising the following steps:
[0087] Mix 100g of methyl acetate and 100g of γ-butyrolactone in a flask, and react for 4 hours at room temperature at about 25°C. After the reaction is completed, continue to add 235g of liquid sodium methoxide into the flask, and continue to stir and react for 1 hour. Quantitatively concentrate 160g of methanol, transfer the material into the acylation kettle, and continue to add 150g of methyl acetate into the acylation kettle, and keep the temperature at about 85°C for 3 hours to carry out the acylation reaction. After the reaction was finished, the temperature was lowered out of the still, neutralized with 85g of acetic acid, and the crude product of sodium acetate and butyrolactone was obtained after filtration. The lactone content is 72% (detected by gas phase GC), and the reaction yield is 92.6%.
Embodiment 2
[0089] A preparation method of α-acetyl-γ-butyrolactone, comprising the following steps:
[0090] Mix 100g of methyl acetate and 100g of γ-butyrolactone in a flask, and react for 4 hours at room temperature at about 25°C. After the reaction, continue to add 250g of liquid sodium methoxide into the flask, and continue to stir and react for 1 hour. Quantitatively concentrate 170g of methanol, transfer the material into the acylation kettle, and continue to add 150g of methyl acetate into the acylation kettle, and keep the temperature at about 85°C for 3 hours to carry out the acylation reaction. After the reaction was completed, the temperature was lowered out of the kettle, neutralized with 88.9g of acetic acid, and the crude product of sodium acetate and butyrolactone was obtained after filtration. The lactone content is 73.6%, and the reaction yield is 94.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com