Peptide mapping method
An analysis method and peptide mapping technology, applied in the field of peptide analysis, can solve the problem of difficult detection of hydrophilic peptides, and achieve the effect of good separation effect and unified method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
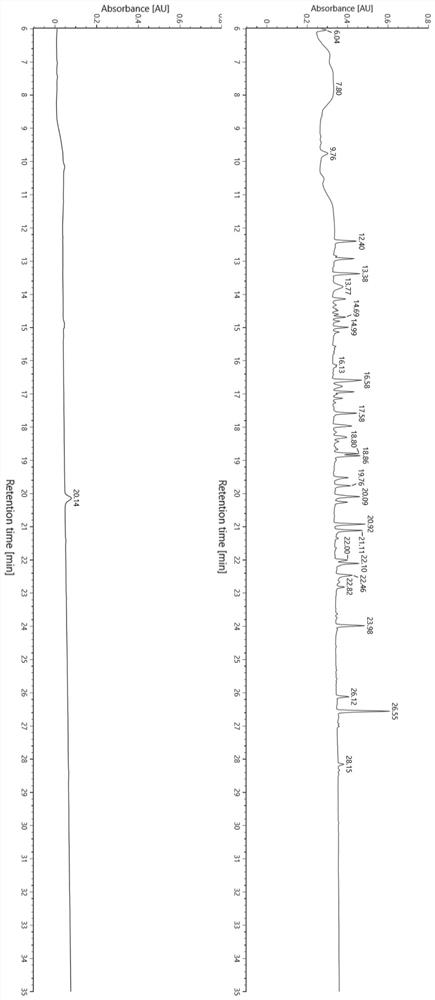
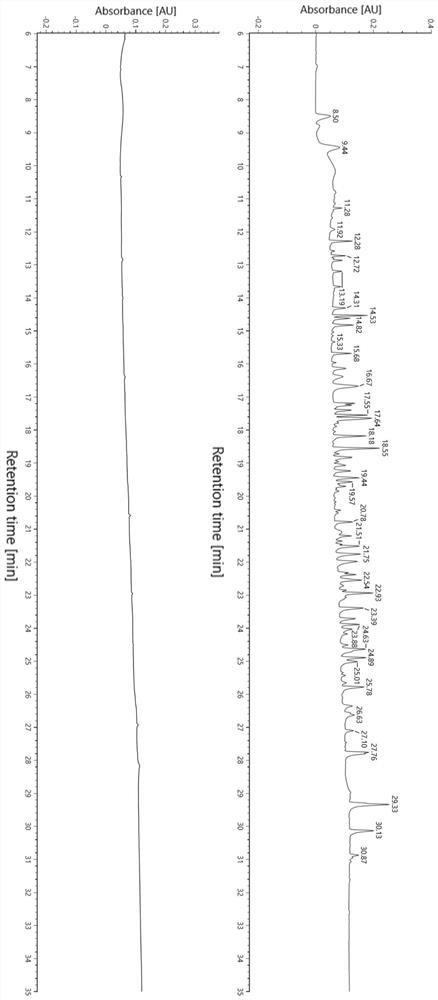
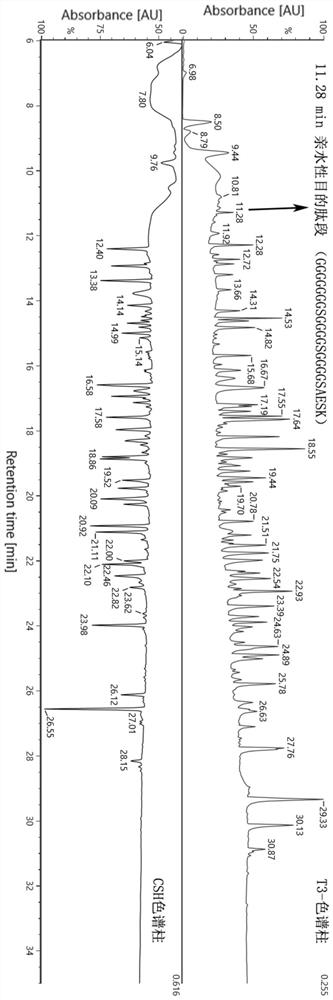
[0070] In this example, a flexible linker (Gly-Gly-Gly-Gly-Ser-Gly-Gly-Gly-Gly-Ser-Gly-Gly-Gly-Gly-Gly-Ser-Ala-Glu-Ser-Lys) was synthesized. The peptide segment was mixed with bovine serum albumin as the test sample, and the effect of the peptide map analysis method in the present invention on the separation and identification of the hydrophilic target peptide segment in the protein sample was verified.
[0071] (1) Experimental reagent consumables and instruments are shown in Table 2 and Table 3.
[0072] Table 2 Experiment reagent consumables
[0073]
[0074] Table 3 Experimental Instruments
[0075] equipment name Instrument model UPLC Waters-H-class plus Constant temperature metal bath Hangzhou Miou Instrument Co., Ltd. DTK200-4 Vortex shaker Hangzhou Miou Instrument Co., Ltd. MIX-25P High speed refrigerated centrifuge Thermo-LEGEND MICRO 21R centrifuge Thermo-ST16R
[0076] (2) Reagent preparation
[0077] 1×PBS so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com