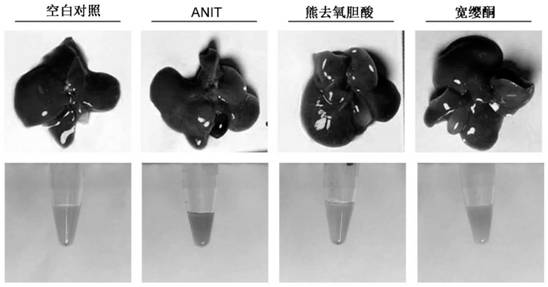
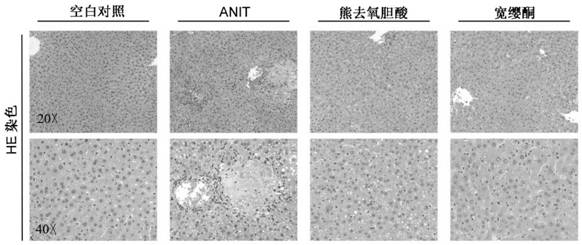
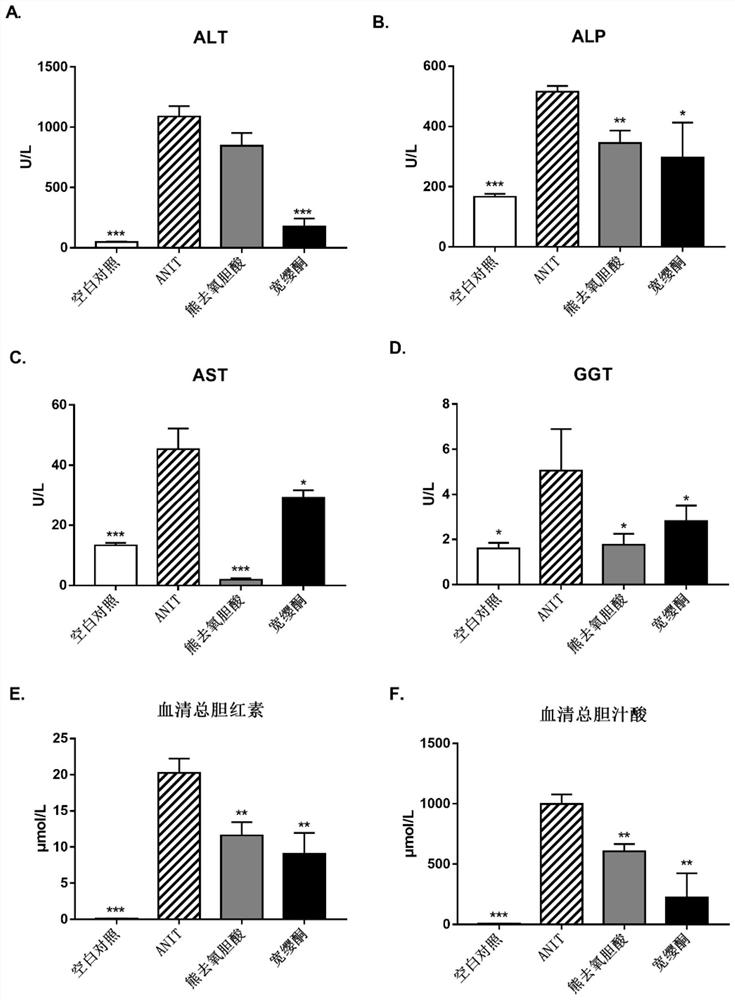
Application of quassin type diterpenoid compound in preparation of medicine for treating cholestatic liver disease
A cholestasis and bitternin technology, applied in the field of medicine, can solve problems such as poor response to UDCA, intolerance to UDCA, and obvious side effects of OCA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0068] As an example of extracting pectinoid diterpenoids from the root of Tongkat Ali, the preparation method comprises the following steps:
[0069] (i) extract Tongkat Ali root with an alcohol solution with a volume concentration of 50% to 90%, and concentrate the extract to obtain the extract;
[0070] (ii) extracting the extract obtained in step (i) to obtain an organic phase extract and an aqueous phase extract respectively;
[0071] (iii) The aqueous phase extract obtained in step (ii) is sequentially eluted with the following solvents on the macroporous adsorption resin column: (a) water or an alcoholic solution with a concentration lower than 40%, (b) 40 -75% alcohol solution, (c) 80-95% alcohol solution;
[0072] (iv) Concentrate the eluate obtained by eluting with (c) 80-95% alcohol solution in step (iii), and use it on a gel chromatography column in a volume ratio of 1:2 to 2:1 The alcohol / water solution was used for elution, and the eluate was collected, concent...
Embodiment
[0090] The content of the present invention is further explained or illustrated by examples below, but these examples should not be construed as limiting the protection scope of the present invention.
[0091] It should be noted that, in the following examples, except for the medicines and reagents whose sources are indicated, other various conventional reagents are known in the art and can be obtained commercially. In addition, each testing instrument used in the present invention is also a conventional instrument known in the art.
[0092] Drugs and Reagents
[0093] Eurycomanone (Eurycomanone, EN): purchased from MCE (MedChemExpress), product number: HY-N5012.
[0094] α-Naphthalene isothiocyanate (ANIT): purchased from Sigma-Aldrich, product number: N4525.
[0095] Ursodeoxycholic acid (UDCA): purchased from MCE (MedChemExpress), product number: HY-13771
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com