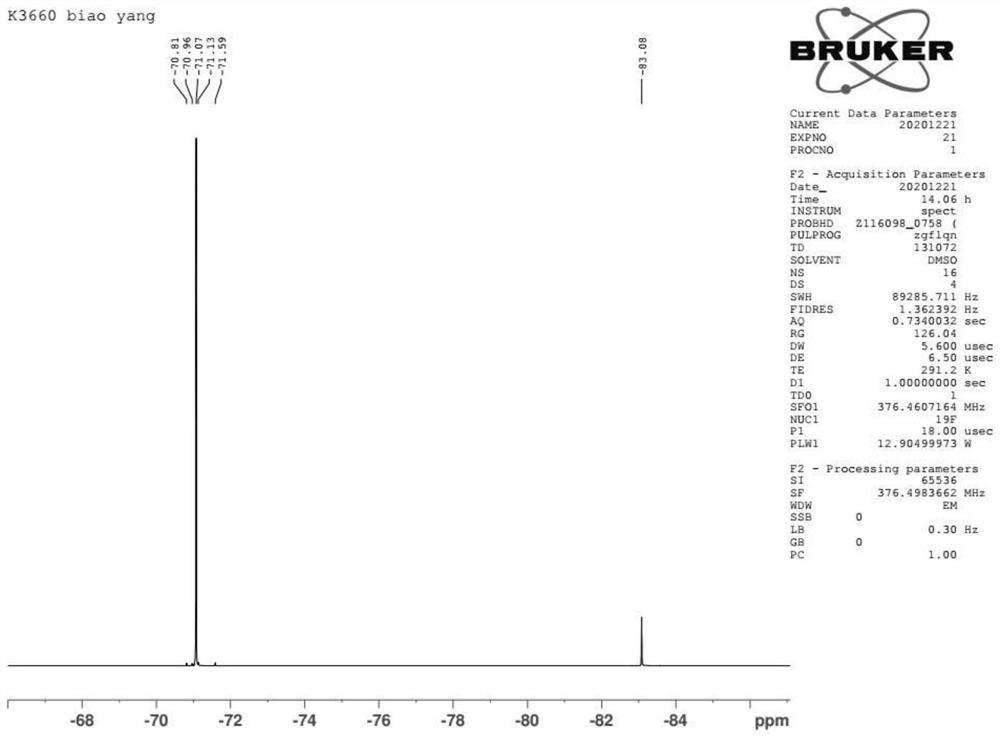
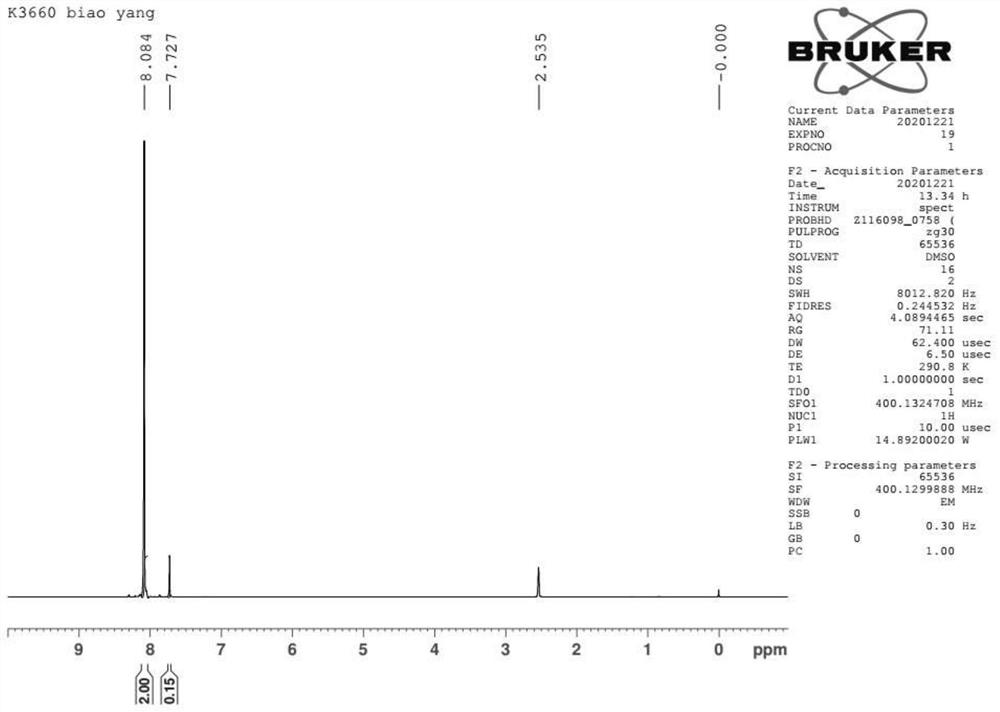
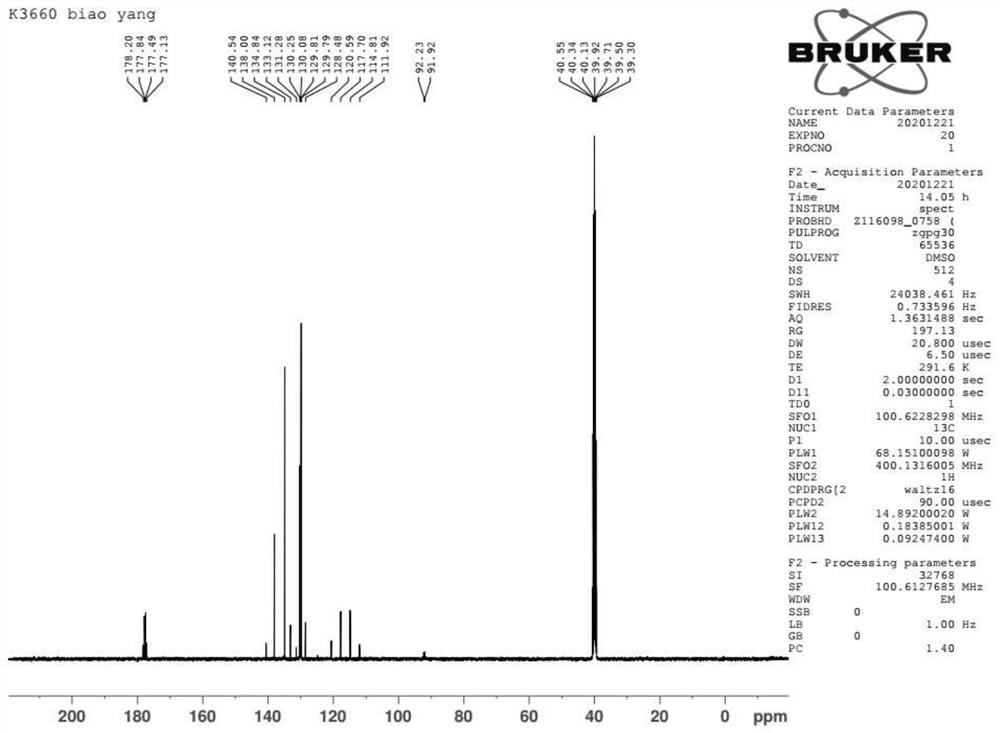
Preparation method of halogenated trifluoroacetyl benzene
A technology of trifluoroacetyl and trichlorotrifluoroacetyl is applied in the preparation of halogenated hydrocarbons, the preparation of organic compounds, the preparation of carbon-based compounds, etc., and can solve complex production operations, low product yield and purity, and few synthesis methods. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The first aspect of the present invention provides a method for preparing 3,4,5-trichlorotrifluoroacetophenone, comprising:
[0051] 1) Diazotization and chlorination of 2,6-dichloro-4-bromoaniline to provide 3,4,5-trichlorobromobenzene;
[0052] 2) 3,4,5-trichlorobromobenzene is subjected to a Grignard reaction to provide 3,4,5-trichlorophenylmagnesium bromide;
[0053] 3) Trifluoroacetylation of 3,4,5-trichlorophenylmagnesium bromide with a trifluoroacetylating reagent to provide 3,4,5-trichlorotrifluoroacetophenone.
[0054] The preparation method of 3,4,5-trichlorotrifluoroacetophenone provided by the present invention may comprise: performing a trifluoroacetylation reaction with 3,4,5-trichlorophenylmagnesium bromide and a trifluoroacetylating reagent , to provide 3,4,5-trichlorotrifluoroacetophenone.
[0055] In the above trifluoroacetylation reaction, the trifluoroacetylating reagent may specifically be one or more combinations of trifluoroacetyldiethylamine, e...
Embodiment 1
[0093] Diazotization, chlorination reaction
[0094] Put 2,6-dichloro-4-bromoaniline (2.4kg, 9.96mol), 30% hydrochloric acid (6kg), water (1kg) into a 20L four-necked flask, start stirring, heat and control the internal temperature to 90-95°C for reaction 1 Hours, cool naturally, add dropwise sodium nitrite aqueous solution (0.76kg sodium nitrite dissolved in 2.4kg water) at an internal temperature of -5 to 0°C, and keep at an internal temperature of -5 to 0°C for 0.5 hours. A solution of the diazonium salt was obtained.
[0095] Take another 20L four-necked flask, put in cuprous chloride (0.4kg, 0.167mol), 30% hydrochloric acid (3.2kg), control the internal temperature at 60-65°C and add the previously prepared diazonium salt solution dropwise, and control the internal temperature at 60-60°C. Continue to keep warm at 65°C for 0.5 hours.
[0096] After the reaction was completed, dichloroethane (4.8 kg) was added, and layers were stirred, the organic layer was washed with wa...
Embodiment 2
[0099] Diazotization, chlorination reaction
[0100] 20L four-neck flask, put 2,6-dichloro-4-bromoaniline (2.4kg, 9.96mol), 30% hydrochloric acid (3kg), toluene (2.5Kg), water (1kg), start stirring, heat and control the internal temperature React at 90-95°C for 1 hour, cool naturally, add tert-butyl nitrite (1232.5g, 11.95mol) dropwise at an internal temperature of -5-0°C, and keep at an internal temperature of 15-20°C for 0.5 hours. A solution of the diazonium salt was obtained.
[0101] Take another 20L four-necked flask, put in cuprous oxide (0.4kg, 0.167mol), 30% hydrochloric acid (3.2kg), control the internal temperature at 60-65°C, add the previously prepared diazonium salt solution dropwise, and control the internal temperature at 60-65°C. °C for 0.5 hours.
[0102] After the reaction, add toluene (4.8kg), stir and separate layers, wash the organic layer with water, separate the water layer, and precipitate the organic layer to obtain 2.5kg of oil.
[0103] The oil w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com