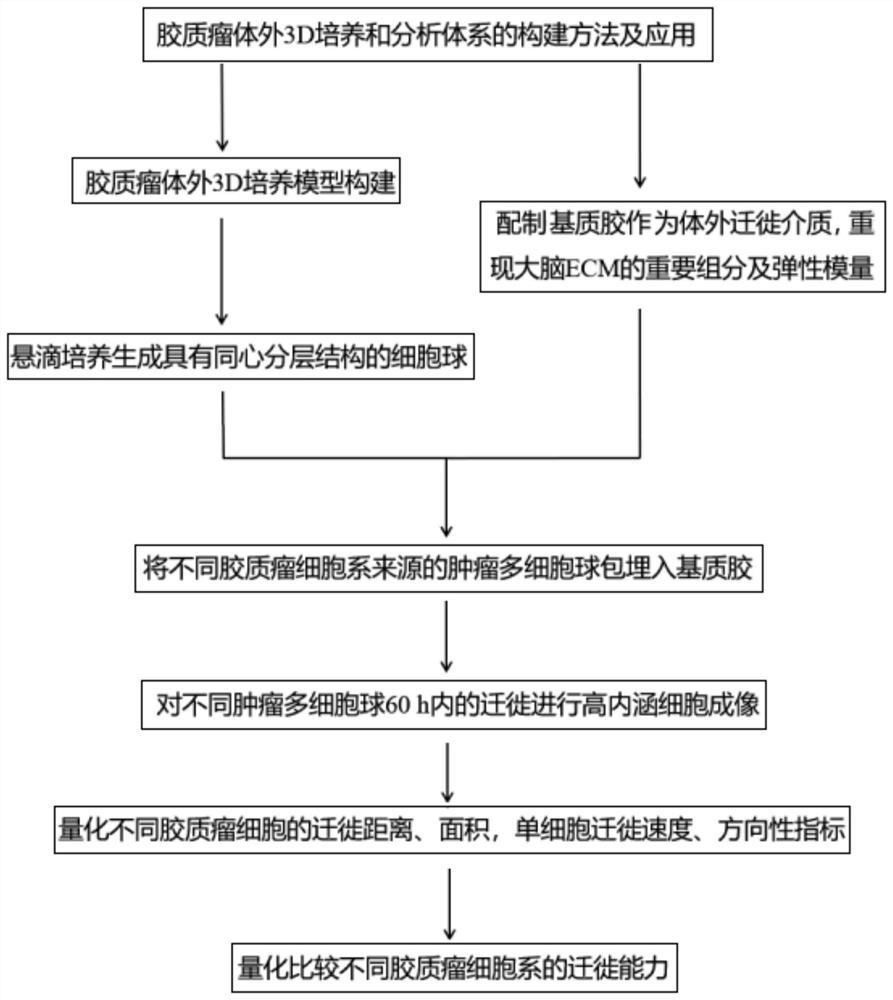
Method for constructing glioma in-vitro 3D culture and analysis system and application
A construction method and technology for glioma, which is applied in the field of constructing an in vitro 3D culture and analysis system for glioma, and can solve the problems of single index for quantifying migration ability and inability to simulate completely.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
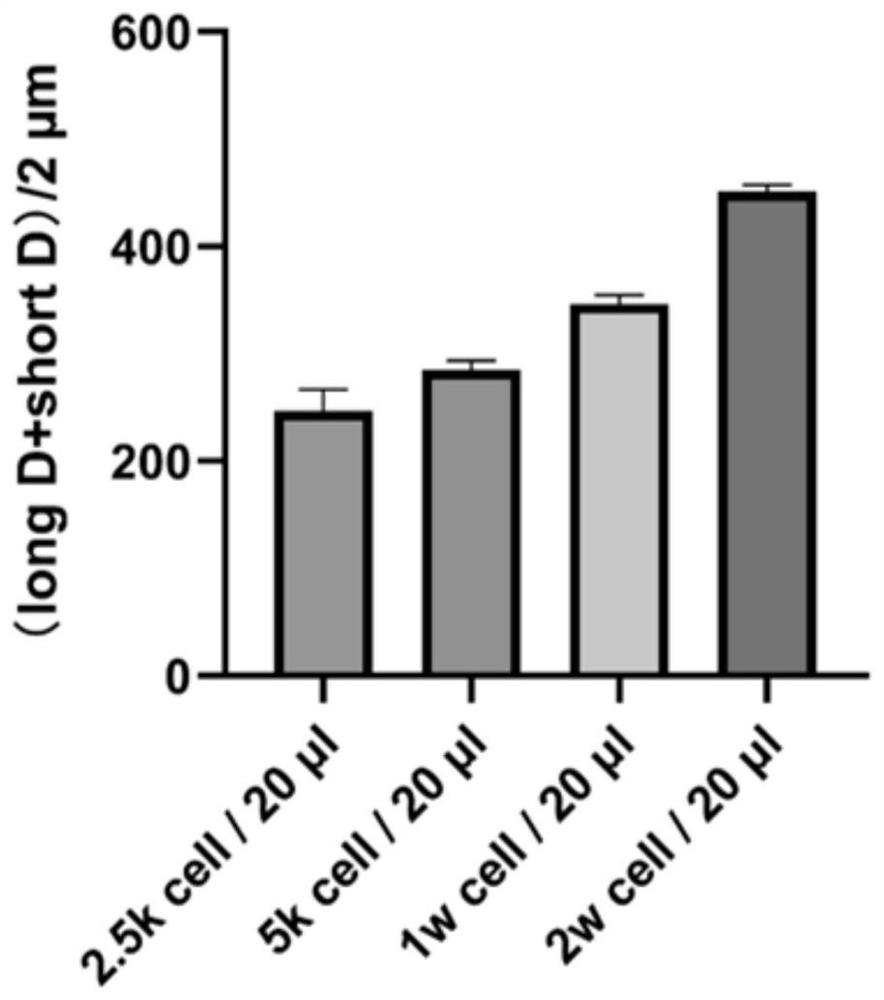
[0035] Example 1. DBTRG, U251 cell lines can produce spheres with necrotic core cell sphere concentration
[0036] 1. The glioblastoma cell lines DBTRG and U251 cryopreserved in liquid nitrogen were recovered and subcultured;
[0037] 2. Obtain the cell suspension of DBTRG and U251 when the cells are subcultured, and use RPMI and DMEM complete medium containing 0.24% methylcellulose to dilute the cell suspension to 2.5kcell / 20μl, 5kcell / 20μl, Concentration of 1wcell / 20μl, 2w cell / 20μl;
[0038] 3. Pipette 20ul of cell suspension into the bottom center of the 24-well plate, cover the 24-well plate and turn the 24-well plate upside down. Finally, add about 5ml of PBS buffer solution to the 24-well plate cover to prevent the cell culture medium from evaporating during the culture process;
[0039] 4. Put the inverted 24-well plate into the cell incubator for culture, and image the cell suspension every 12 hours within 72 hours to observe the sphere formation of tumor multicellu...
Embodiment 2
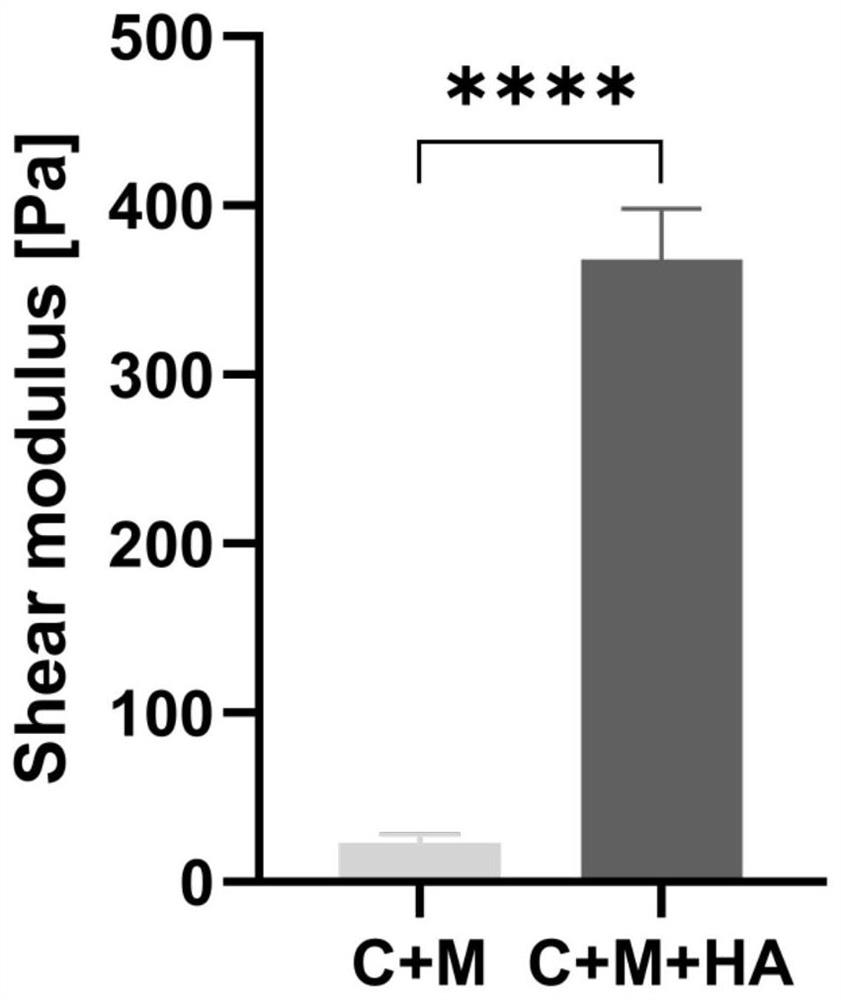
[0040] Example 2. Quantification of elastic modulus parameters of matrix gels without and with hyaluronic acid
[0041] (1) Prepare a mixed gel of 0.5mg / ml Collagen I+3mg / ml Matrigel:
[0042] 1. Take out Collagen I and Matrigel matrigel, which are packed in 2ml centrifuge tubes and stored in refrigerators at 4°C and -20°C respectively, and store them in crushed ice (the whole process is performed on ice);
[0043] 2. Use 10×PBS, 1N NaOH, dH2O to dilute 3mg / ml Collagen I to 1mg / ml for later use. The specific method is as follows:
[0044] vt = total volume of collagen gel required, required collagen volume (v1) = (final concentration of collagen) x (total volume (vt)) divided by initial collagen concentration.
[0045] Required volume of 10xPBS (v2) = total volume (vt) / 10;
[0046] Required 1N NaOH volume (v3) = (v1) × 0.025;
[0047] Required dH2O volume (v4) = (vt) – (v1+v2+v3).
[0048] 3. Use RPMI and DMEM simple medium to dilute Matrigel to two concentrations of 6mg / m...
experiment example 1
[0057] Experimental example 1. DBTRG, U251 cell spheres embedded in 0.5mg / ml Collagen I+3mg / ml Matrigel+HA for migration
[0058] 1. Take a 96-well plate, add 50 μl of mixed gel to each well as an in vitro migration medium, embed DBTRG and U251 tumor multicellular spheres in it, and place them in a cell culture incubator for 30 minutes until the gel solidifies.
[0059] 2. Place the 96-well plate in the high-content cell imaging system, and perform imaging every 30 minutes for 60 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com