Spray compositions of chitosan for wound healing
A technology of composition and chitosan, which is applied in the direction of drug delivery, aerosol delivery, medical preparations of non-active ingredients, etc., can solve the problems of poor adhesion of wounds, inconvenient storage of large areas, large use area, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
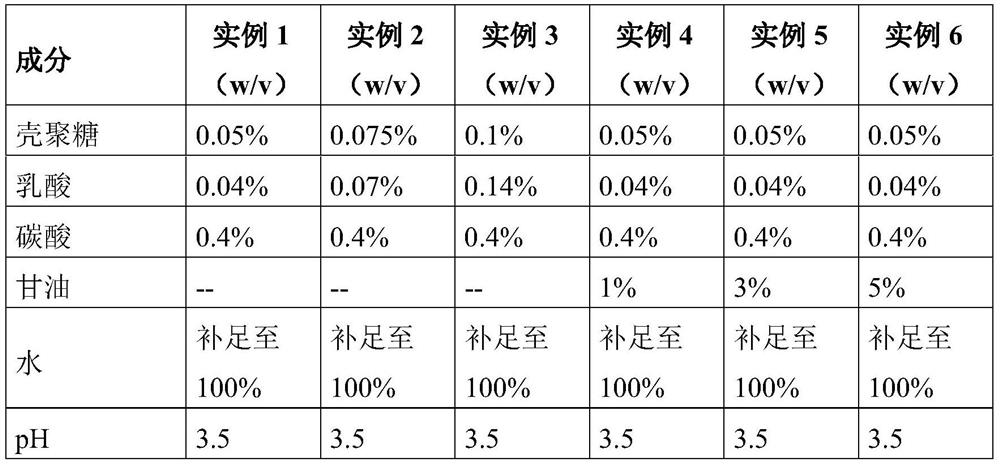
example 1-3
[0088] The preparation method of example 1-3
[0089] a. Collect 70% of the batch of water for injection into a suitable manufacturing container.
[0090] b. Add and disperse chitosan in step a.
[0091] c. Slowly add the required amount of 1 molar lactic acid until the chitosan is completely dissolved.
[0092] d. Make up volume to 100% batch with water for injection
[0093] e. The solution was filtered through 0.2μ and cooled to below 5°C.
[0094] f. Carbonate the solution and immediately fill into containers and close.
[0095] NOTE: If filled non-sterile, the product should be sterilized by gamma irradiation or other suitable sterilization method.
example 4-6
[0096] The preparation method of example 4-6
[0097] a. Collect 70% of the batch of water for injection into a suitable manufacturing container.
[0098] b. Add and disperse chitosan in step a.
[0099] c. Slowly add the required amount of 1 molar lactic acid until the chitosan is completely dissolved.
[0100] d. Add the portioned amount of glycerin to step c.
[0101] e. Make up volume to 100% batch with water for injection.
[0102] f. The solution was filtered through 0.2μ and cooled to below 5°C.
[0103] g. Carbonate the solution and immediately fill into containers and close.
[0104] NOTE: If filled non-sterile, the product should be sterilized by gamma irradiation or other suitable sterilization method.
example 7
[0105] Example 7: Excision Wound Healing Study Protocol
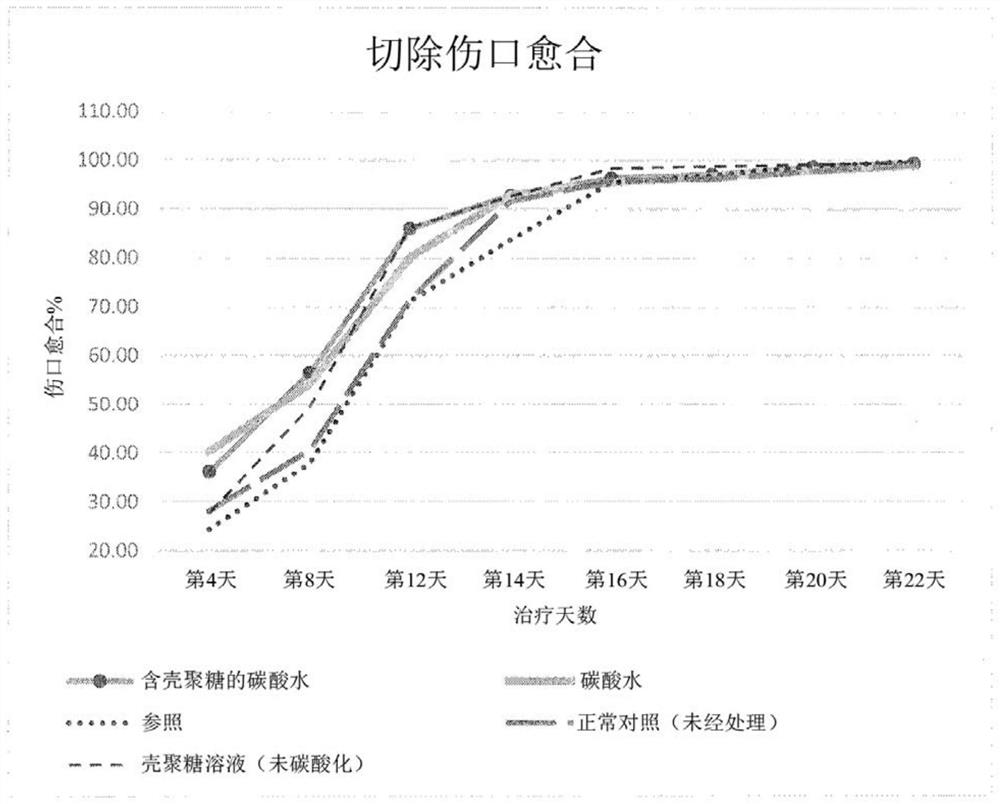
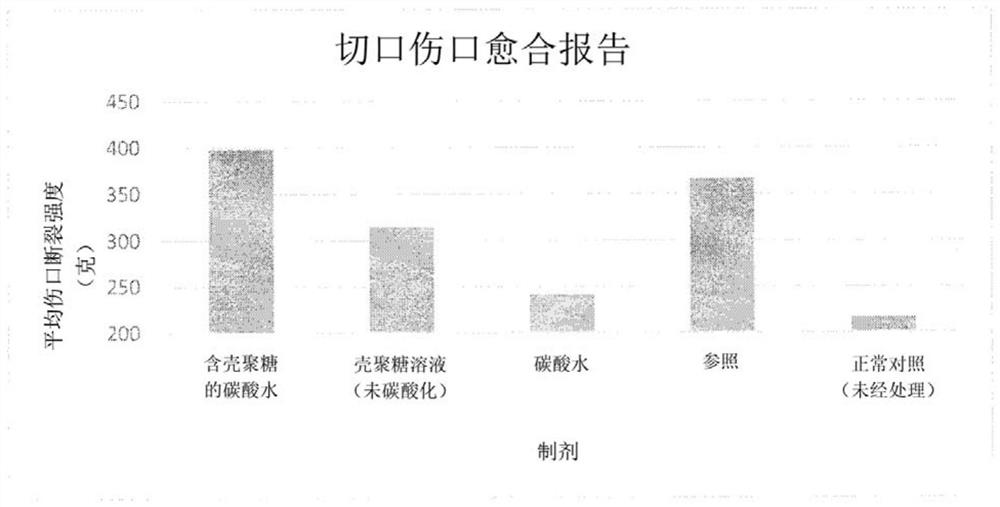
[0106] Anesthetize the rat with ketamine (30 mg / kg, ip) and mark about 500 mm with a standard ring on the back of the rat 2 area. The marked full-thickness skin is then carefully excised. Treatment started on day 1 and continued until day 22. Wounds were traced on OHP sheets, calculated on graph paper by superimposition on the day of wounding and at subsequent intervals of 4 days until day 12, and every other day thereafter until healing.
[0107] Changes in the wound area were measured regularly, and the wound contraction rate was calculated at the end of the study. The results are shown in Table 1 and figure 1 mentioned.
[0108] Table 1
[0109] Excision wound area - % inhibition:
[0110]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com