Method for producing intestinal cells from pluripotent stem cells
A production method and enterocyte technology, which can be applied to non-embryonic pluripotent stem cells, artificially induced pluripotent cells, and gastrointestinal cells, etc., and can solve problems such as small number of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0097] Hereinafter, although an Example demonstrates this invention in detail, this invention is not limited to these Examples.
[0098] (A) result
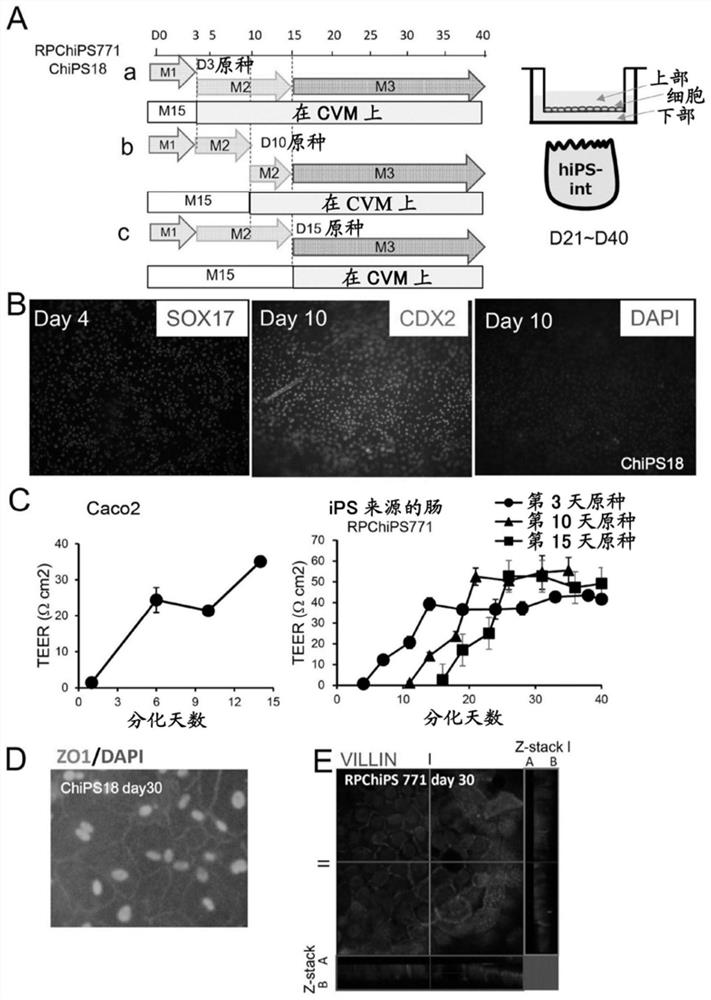
[0099] (1) Collagen Vitrigel supports the differentiation of human iPSCs into intestinal cells characterized by membrane formation and expression of intestinal markers.
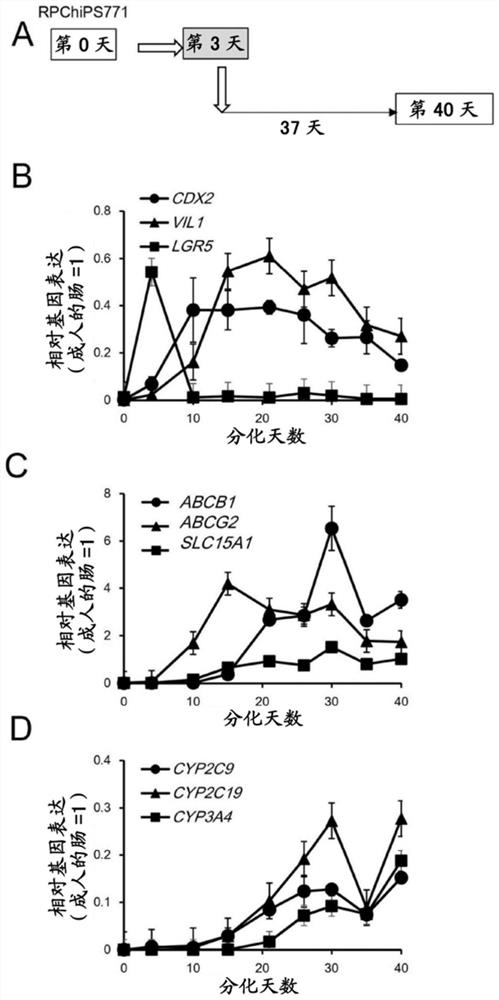
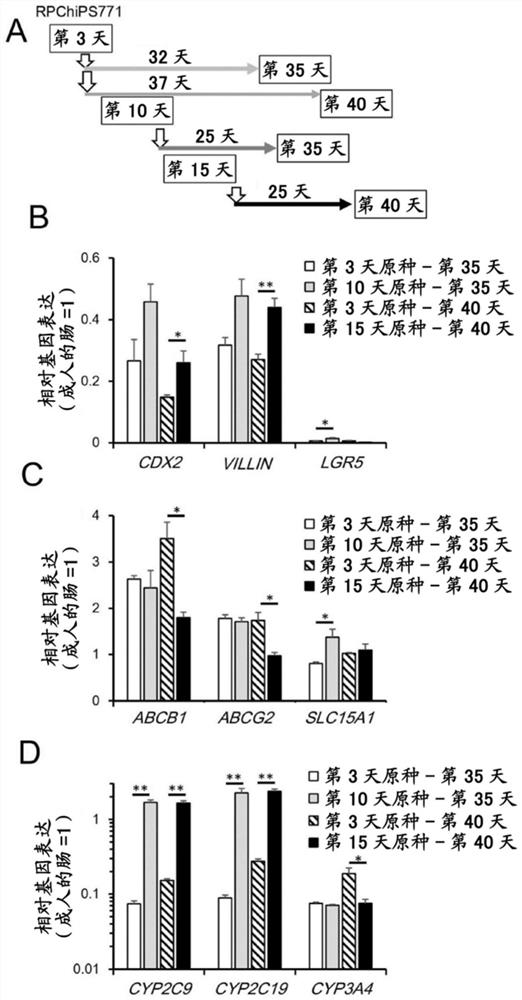
[0100] In an attempt to induce the differentiation of human iPS cells into mature intestinal cells, the inventors initially induced embryonic endoderm (DE) cells from human iPSCs on M15 cells. Day 3 (D3) DE cells were dissociated, replaced on CVM inserts, and cultured in M2 medium until day 15. Then, the medium was exchanged into a mature medium (M3, M3-1, or M3-2), and cultured until the 40th day ( figure 1 A. Figure 6 A). Alternatively, day 3 DE cells were repeatedly plated on iMatrix511 precoated plates, cultured in M2 medium until day 10, and subsequently cryopreserved as a day 10 intestinal precursor cell stock. Freeze-thaw the frozen-preserved day 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com