Parecoxib derivative as well as preparation method and application thereof
A compound and solvate technology, applied in the field of pharmaceutical compounds, can solve problems such as precipitation, and achieve the effect of good patient compliance and operational safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
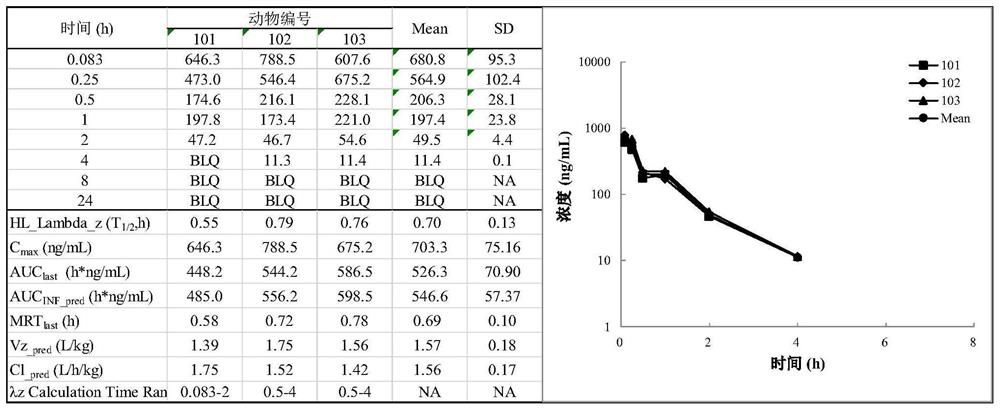
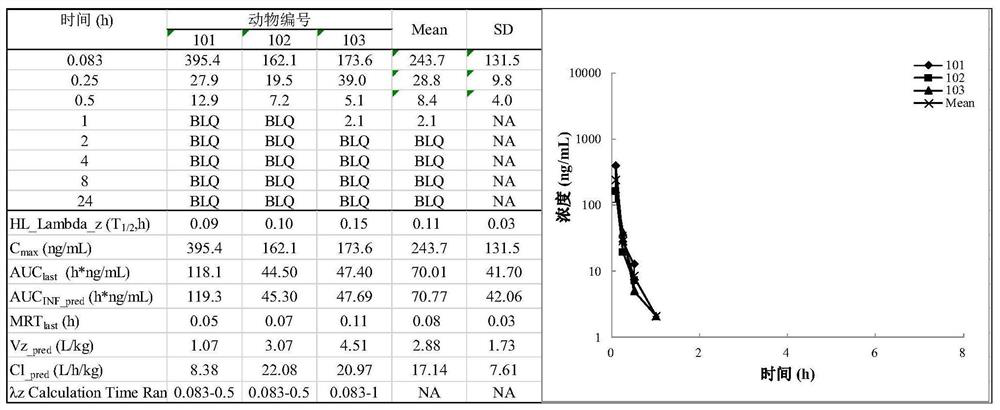
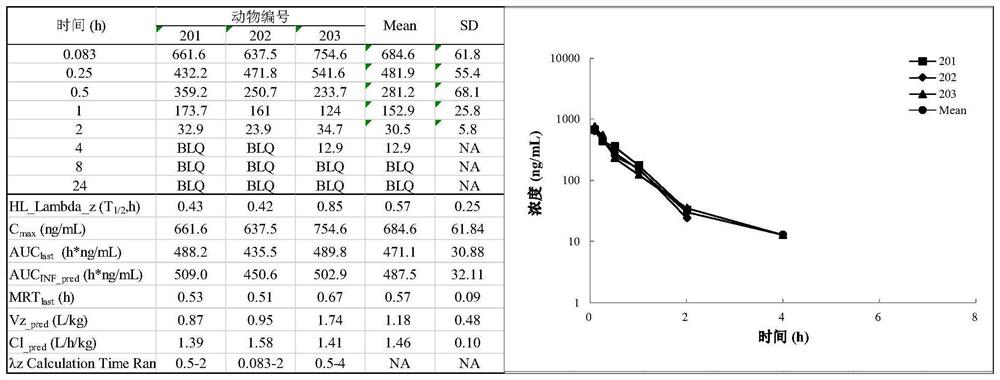
Image
Examples
Synthetic example 1
[0096]
[0097] K 2 CO 3 (375 mg, 2.7mmol, 5.0eq), methyl chloroacetate (1.16g, 10.8mmol, 20eq), and stirred at room temperature for 18 hours. with H 2 The reaction mixture was diluted with O (20 mL), extracted with EtOAc (20 mL*2). The combined organic phases were washed with saturated brine (15 mL), washed with Na 2 SO 4 After drying, filter and concentrate in vacuo. The obtained crude product residue was purified by TLC (PE / EA=1.7:1) to obtain 50 mg of crude product, which was purified by prep-HPLC (0.1% HCOOH / H in ACN 2 O) Further purification gave white solid compound 1 (16.65 mg, yield 6%). LCMS: (M+H)+: 443.1
[0098] 1 H-NMR (400MHz, CDCl 3 )δ=8.00(m,2H),7.38(m,7H),5.91(s,2H),2.61(q,J=7.2Hz,2H),2.52(s,3H), 2.08(s,3H), 1.10(t,J=7.2Hz,3H).
Synthetic example 2
[0100]
[0101] K 2 CO 3 (746 mg, 5.41 mmol, 1.54 eq), chloromethyl isopropyl carbonate (4 mL), and stirred at room temperature for 18 hours. with H 2 The reaction mixture was diluted with O (30 mL), extracted with EtOAc (40 mL*3). The combined organic phases were washed with saturated brine (30mL*3), washed with Na 2 SO 4 It was dried, filtered, and concentrated to obtain a crude residue, which was purified by TLC (EtOAc:PE=1:2) to obtain white solid Example 2 (104.4 mg, yield 33.73%). LCMS: (M+H)+: 487.2
[0102] 1 H-NMR (400MHz, DMSO-d6) δ=8.01-7.94(m,2H),7.50-7.39(m,5H),7.35-7.30(m,2H),5.91(s,2H), 4.87-4.71( m,1H),2.65(dd,J=7.1Hz,2H),2.49(s,3H),1.23(d,J=6.2Hz,6H),0.92(t,J=7.2Hz,3H).
[0103] Synthetic Example 3.
[0104]
[0105] Add DIPEA (174 mg, 1.35mmol, 2.5eq), methyl chloromethyl carbonate (134.4mg, 1.08mmol , 2.0eq), the reaction was stirred at room temperature for 18 hours. Spin dry with H 2 The reaction mixture was diluted with O (3 mL), extracte...
Embodiment 1
[0113] Synthesize following compound 5-28 with reference to embodiment 1
[0114] List of specific examples
[0115]
[0116]
[0117]
[0118]
[0119] Also included within the scope of the present invention is a pharmaceutical combination comprising said active compound in admixture with one or more non-toxic pharmaceutically acceptable carriers and / or diluents and / or adjuvants (hereinafter collectively referred to as "carrier" materials) substances, which may also contain other pharmaceutically active ingredients as required. The active compound of the present invention can be administered orally or externally, preferably in the form of a drug combination suitable for the administration route and at an effective dose required for treatment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com