Freeze-drying protection liquid, freeze-drying method and application of erythrocyte membrane fragment
A technology of freeze-dried protective solution and red blood cell membrane, which is applied to freeze-dried cell membrane fragments and its application in the detection of blood type reversal. Many other problems to achieve the effect that is conducive to standardization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of erythrocyte membrane fragments
[0039] The red blood cell debris preparation process is as follows:
[0040] 1) Take 3 mL of anticoagulated mixed whole blood (taken from 6-10 blood samples of type A (or other blood types), add it to a 15 mL centrifuge tube filled with 10 mL of 0.01mol / L PBS (pH7.2), and Centrifuge at 3000r / min for 5min, discard the supernatant and the white blood cell and platelet layer under the supernatant, and obtain about 1.5mL packed red blood cells;
[0041] 2) Wash 3 times with 4°C pre-cooled 0.01 mol / L PBS (pH7.2) equivalent to 3 times the volume of packed red blood cells, and centrifuge at 5000 r / min for 15 minutes at 4°C each time;
[0042] 3) Add 4°C pre-cooled 0.01 mol / L PBS (pH7.2) at a ratio of V:V = 40:1 and mix with the sediment, place at 4°C for 2 hours, then centrifuge at 9000r / min for 20 minutes, discard clear;
[0043] 4) Repeat step 3) 4 more times until no red blood cells are visible to the naked eye, a...
Embodiment 2
[0045] Example 2 Preparation of Lyoprotectant Solution
[0046] Using water for injection, prepare 3 kinds of lyoprotectant solutions according to the following components and concentrations:
[0047] 1#: 20mM glucose, 10mM lactose, 140mM trehalose, 39mM NaCl, 5.3mM KCl, 0.125mM KH 2 PO 4 , 1.2mM Na 2 HPO 4 ;
[0048] 2#: 10mM glucose, 12mM lactose, 141mM trehalose, 31mM NaCl, 4.3mM KCl, 0.115mM KH 2 PO 4 , 1.1mM Na 2 HPO 4 ;
[0049] 3#: 15mM glucose, 10mM lactose, 131mM trehalose, 35mM NaCl, 5.0mM KCl, 0.115mM KH 2 PO 4 , 1.1mM Na 2 HPO 4 .
[0050] In the description below, the corresponding lyoprotectant solutions are represented by corresponding numbers (1#, 2# or 3#).
Embodiment 3
[0051] Example 3 Lyophilization of erythrocyte membrane fragments
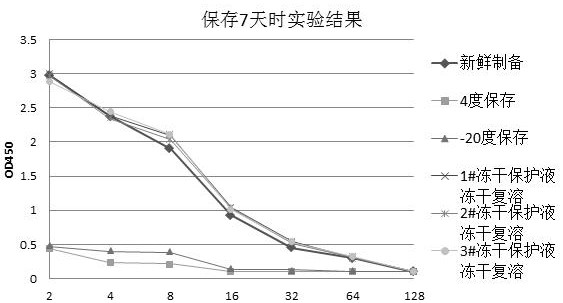
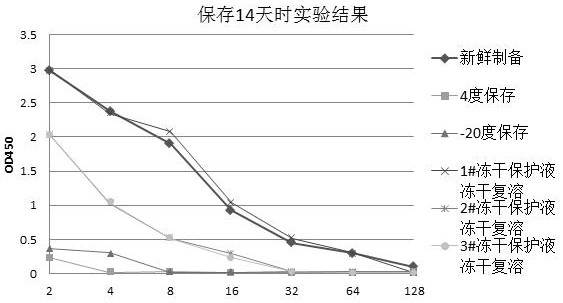
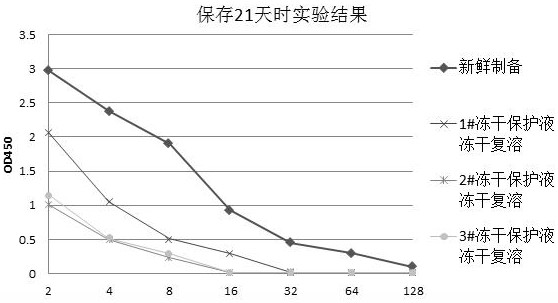
[0052] The fresh erythrocyte membrane fragments prepared in Example 1 and the lyoprotectants prepared in Example 2 were mixed according to a volume ratio of 1:9, and added to a vial with a volume of 5 mL, so that the liquid content of each vial was 1 mL. Put the vial in a refrigerator at 4 degrees Celsius for 30 minutes, then drop it to -70 degrees Celsius at a rate of 10 degrees Celsius / minute, and keep it at this temperature for more than 2 hours to freeze the freeze-dried product. Then start to vacuumize and dry once, so that the partition temperature and pressure of the lyophilizer are kept at about -50 degrees Celsius and 2-4 Pa respectively, and the time is 18 hours. Finally, secondary drying is performed, during which the temperature of the partition is set at 20 degrees Celsius, the pressure is about 2-4 Pa, and the duration is 10 hours to obtain freeze-dried erythrocyte membrane fragments.
[0053] F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com