Recombinant lentivirus vector for treating beta-globulin afunction, preparation method and application
A technology of recombinant lentivirus and loss of function, which is applied in virus/bacteriophage, botanical equipment and methods, plasma globulin/lactoglobulin, etc., can solve the problems of high cost, complicated production process, high price, etc., and achieve increased safety , Improving the efficiency of gene transduction and reducing the cost of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0057] 1. Test method
[0058] 1. Construction of recombinant lentiviral vector
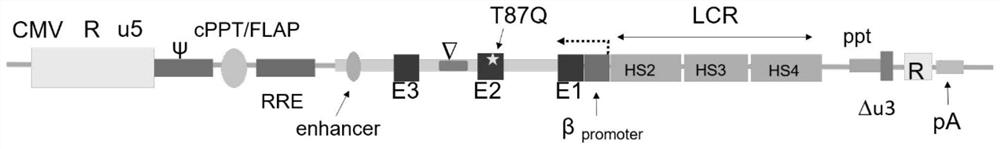
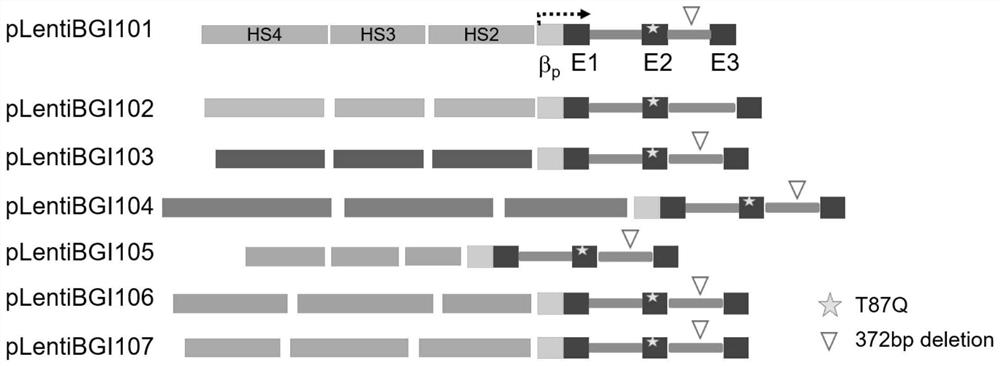
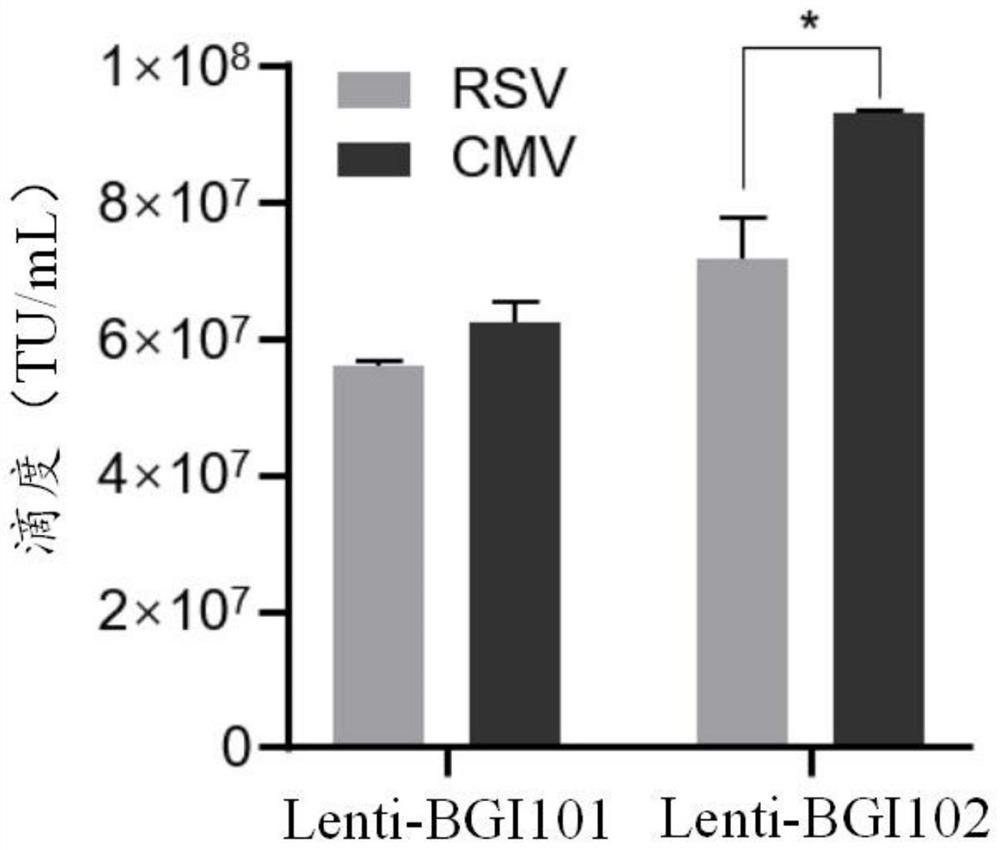
[0059] The recombinant lentiviral vector in this example, such as figure 1 As shown, it is mainly composed of the following elements: left lentiviral long terminal repeat (5'LTR), lentiviral reverse response element (RRE), central polypurine sequence (cPPT), HBB locus regulatory sequence (LCR), p. β-globin gene sequence with threonine at position 87 mutated to glutamine (HBB T87Q ), and the right lentiviral long terminal repeat (3'LTR). The long terminal repeat sequence (LTR) of the lentivirus can be further divided into three regions U3, R and U5. The U3 region of the left long terminal repeat sequence (5'LTR) of the viral vector used in this example is replaced by a Strong heterologous promoters. In this case, the cytomegalovirus promoter (CMV promoter) and the RSV promoter were used for experiments to improve the transcription and packaging efficiency of the viral genome sequence. The U3 r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com