Modified immunologic effector cell and preparation method thereof
A technology of immune effector cells and cells, applied in genetically modified cells, cells modified by introducing foreign genetic material, and other methods of inserting foreign genetic material, etc., can solve the problem of rapid rejection by the host immune system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
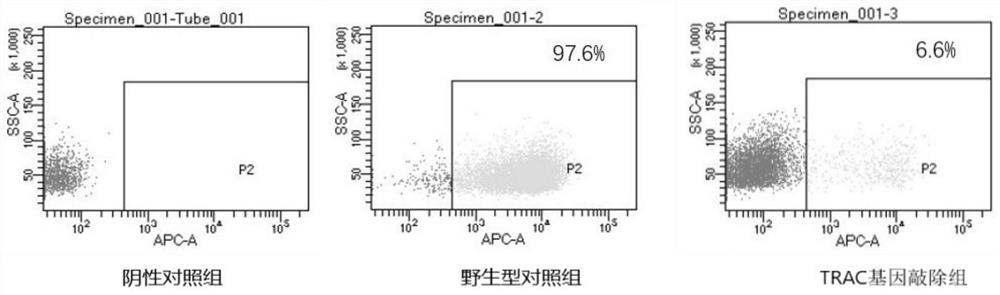
[0275] 1. A modified immune effector cell, wherein the expression and / or activity of the TRAC gene and the HLA-A gene are down-regulated compared with the expression and / or activity of the corresponding gene in the corresponding cell without said modification, and the expression and / or activity of the B2M gene Expression and / or activity is not downregulated, and expression and / or activity of the CIITA gene is not downregulated.
[0276] 2. The immune effector cell according to embodiment 1, wherein the modification results in down-regulation of the expression and / or activity of two genes, wherein the two genes consist of the TRAC gene and the HLA-A gene.
[0277] 3. The immune effector cell according to any one of embodiments 1-2, wherein compared with the corresponding wild-type cells, the expression and / or activity of the TRAC gene and the HLA-A gene are down-regulated, and the expression and / or activity of the B2M gene are down-regulated. / or activity is not down-regulated,...
Embodiment 1
[0325] Example 1 Design guide RNA
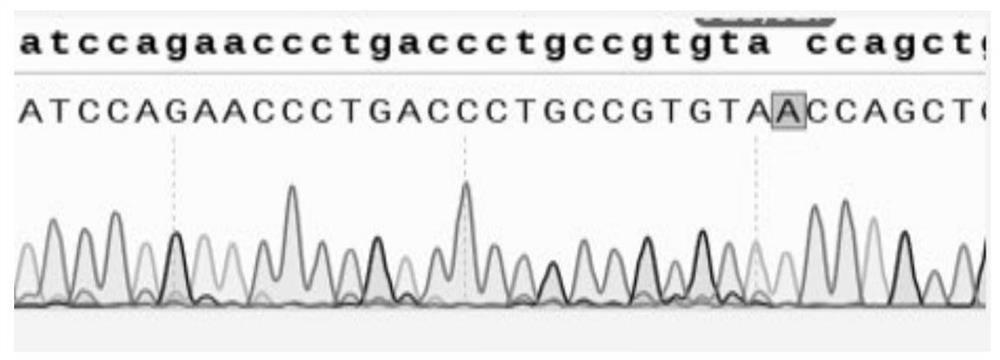
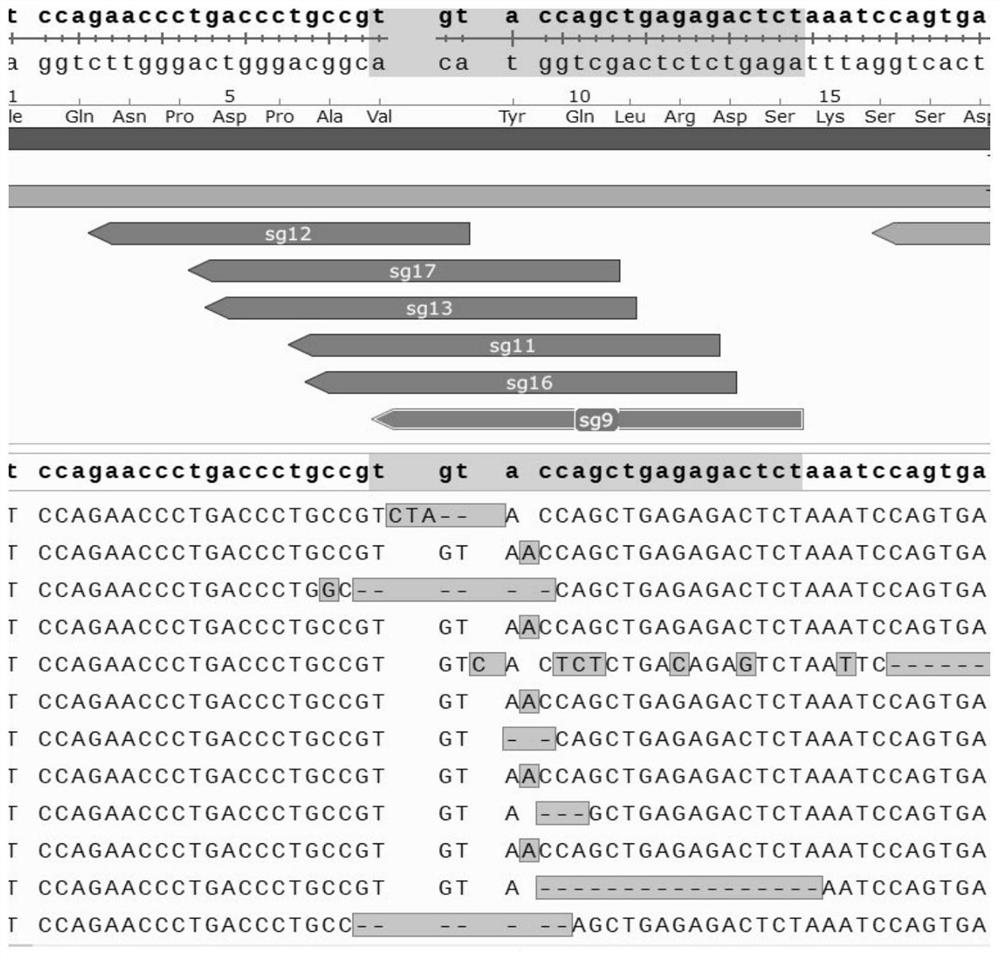
[0326] Through the website https: / / www.ncbi.nlm.nih.gov / , find and download the corresponding gene sequence (as shown in SEQ ID No.55-63), and use the SnapGene software to open the gene sequence, which can be used in different exosomes of the target gene Design sgRNA on sub. The non-limiting design principle of the sgRNA of the CRISPR / Cas9 system used in this example is: 5'-NNN(20)-NGG-3', NGG is called protospacer adjacent motif (PAM), wherein , N represents A, T, C or G. Since many sgRNAs can be designed on the same exon, and sgRNAs consisting of 20 nucleotide sequences may appear repeatedly in the genome, use the website http: / / crispr.cos.uni-heidelberg.de to Carry out the design and evaluation of sgRNA, paste the exon sequence to this website, the website designs sgRNA and conducts prediction evaluation, the higher the score in the evaluation, it means that there may be higher editing efficiency and lower off-target risk, from which S...
Embodiment 2
[0327] Example 2: CD3 + T cell preparation
[0328] (1) Isolation of PBMCs from peripheral blood
[0329] Peripheral blood was collected from healthy donors and diluted 1:1 with PBS buffer. Into a new 50ml centrifuge tube, first add cell separation solution (Ficoll) with 1 / 3 of the diluted blood volume, then add the blood cell dilution solution very slowly along the tube wall, and centrifuge at 800g for 20min at room temperature (the centrifuge is set to speed up 1, speed down 0). After centrifugation, the liquid in the centrifuge tube is divided into PBS and serum layer, white blood cell layer, lymphocyte separation solution, and red blood cell layer from top to bottom. Remove the PBS and serum layer, move the white blood cell layer to a new 50ml centrifuge tube, add PBS to 40ml to wash the cells, and centrifuge at 450g for 10min. After centrifugation, the supernatant was discarded to obtain peripheral blood mononuclear cells. Cell counts were performed after the cells w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com