Application of irisin in preparation of medicine for treating aplastic anemia
A technology for aplastic anemia and irisin, applied in the field of medicine, can solve problems such as the application of aplastic anemia without irisin, and achieve good histocompatibility
Active Publication Date: 2021-07-09
THE SECOND HOSPITAL OF SHANDONG UNIV
View PDF3 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0004] Irisin can be produced by various tissue cells such as skeletal muscle, cardiac muscle, liver, kidney, nerve, fat, pancreas, etc. Current research mainly focuses on the application of irisin in improving obesity and other metabolic diseases. Currently, there is no irisin The application of Suin in the treatment of aplastic anemia
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
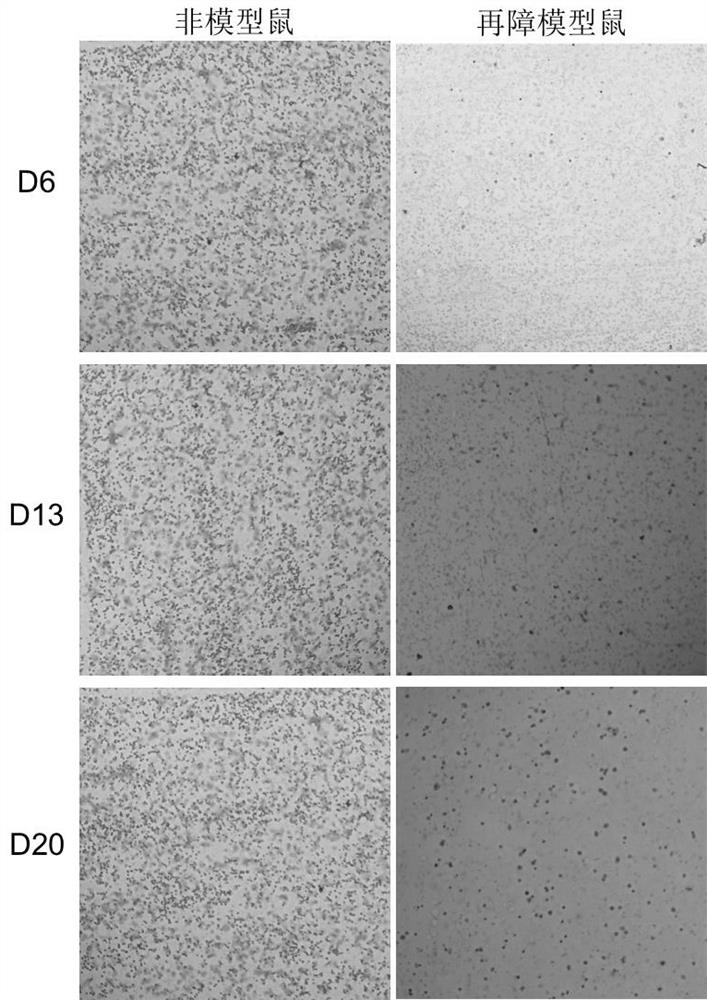
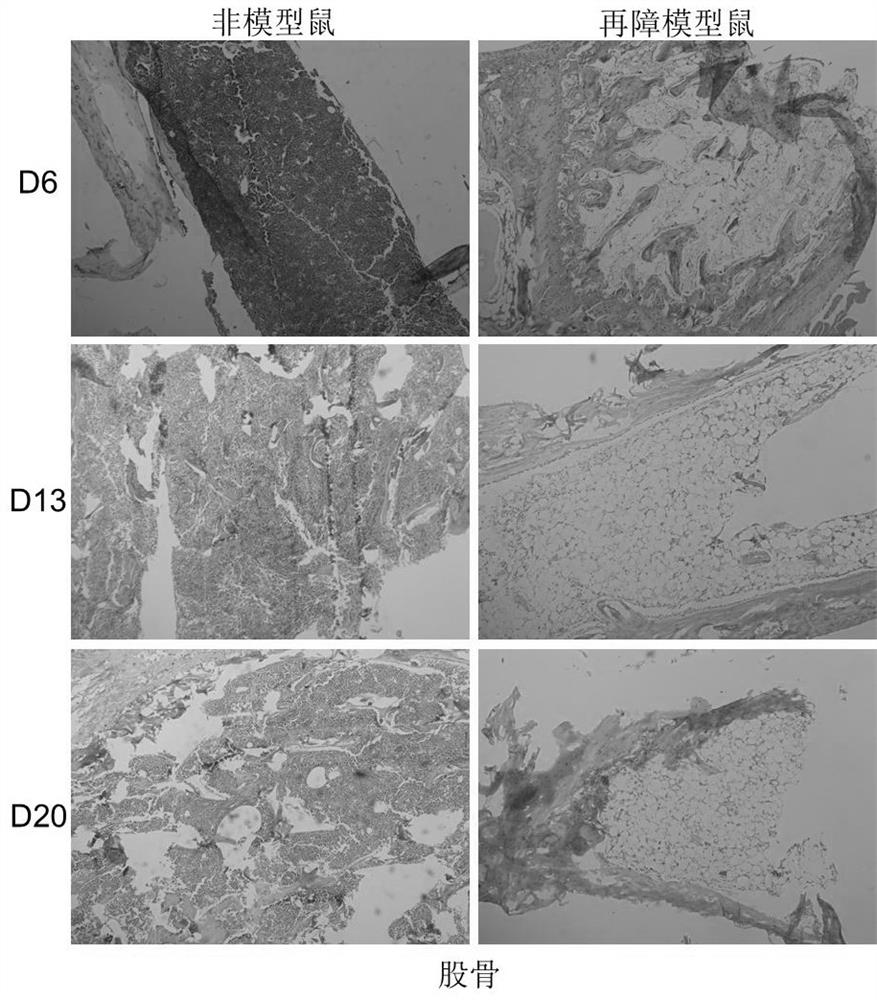
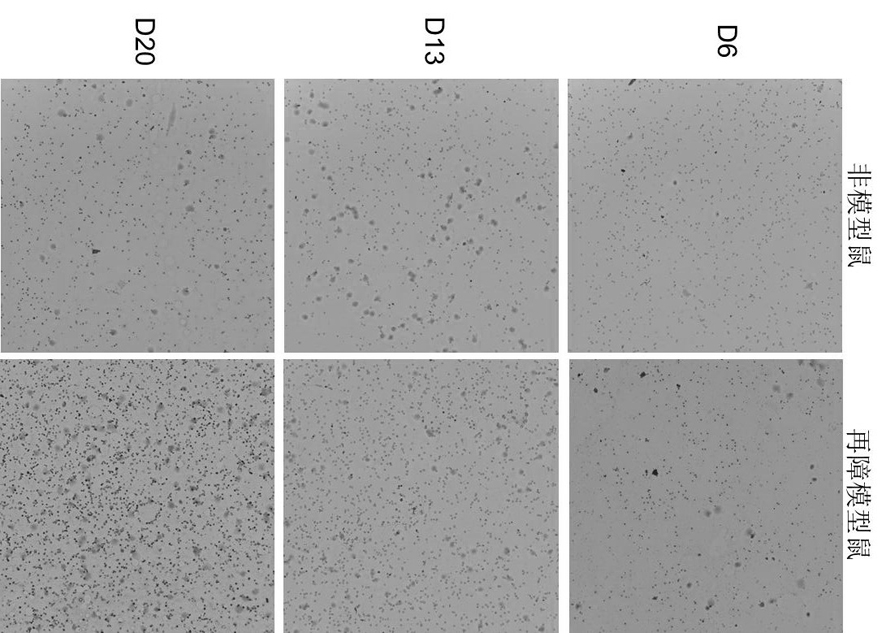
[0025] 1. Establishment of aplastic anemia model in mice
[0026] Using 6Gy mouse total body irradiation (TBI) combined with tail vein lymphocyte infusion, the pathological features of pancytopenia and bone marrow dysplasia similar to human hematopoietic stem cell failure were reproduced in mice.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses an application of irisin in preparation of a medicine for treating aplastic anemia. The administration mode is intraperitoneal injection administration, and the administration dosage is 0.5 [mu]g / g; according to the application of the irisin in preparation of the medicine for treating aplastic anemia, due to the fact that the irisin is free of species specificity and good in histocompatibility, immunological rejection on aplastic anemia patients is avoided; the irisin is applicable to immune cell mediated aplastic anemia and non-immune mediated acute aplastic anemia, especially hereditary anemia, a new intervention target is provided for preparation of aplastic anemia drugs, and application of the irisin to development of various types of aplastic anemia-related drugs provides significant significance for treatment of aplastic anemia. A brand new choice and thought are provided for research of aplastic anemia drugs, and the selection field of aplastic anemia drugs is widened.
Description
technical field [0001] The invention relates to the technical field of medicine, in particular to the application of irisin in the preparation of medicines for treating aplastic anemia. Background technique [0002] Aplastic anemia is a disease caused by a severe lack of hematopoietic stem cells and progenitor cells and bone marrow hematopoietic failure, leading to peripheral pancytopenia in patients. The main clinical manifestations are anemia, hemorrhage and infection. Bone marrow lesions are mainly caused by the reduction of hematopoietic tissue and the reduction of the total volume of red bone marrow, which is replaced by adipose tissue. Therefore, the replacement of hematopoietic cells by fat is a unique pathological feature of aplastic anemia. Almost all sporadic aplastic anemias, especially severe and acute ones, are thought to be immune cell-mediated. Therefore, the current treatment of aplastic anemia is mostly immunosuppressive therapy, such as antithymocyte globu...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K38/22A61P7/06A61P7/00
CPCA61K38/22A61P7/06A61P7/00
Inventor 唐东起郑成云孔德晓李慧
Owner THE SECOND HOSPITAL OF SHANDONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com