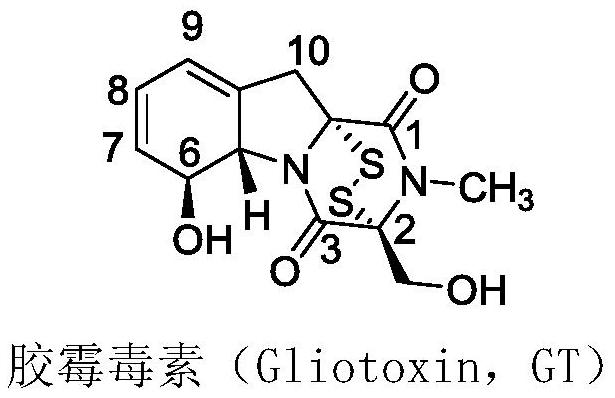
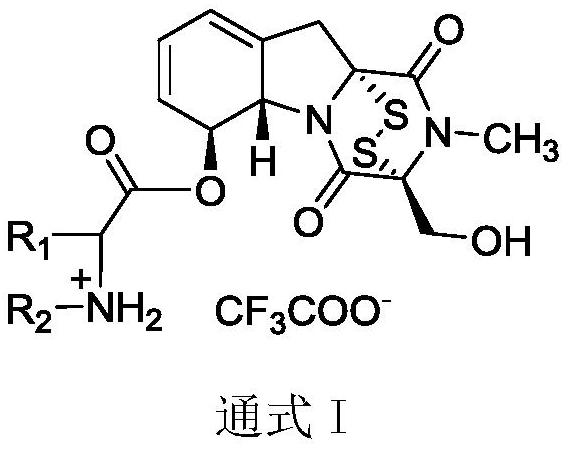
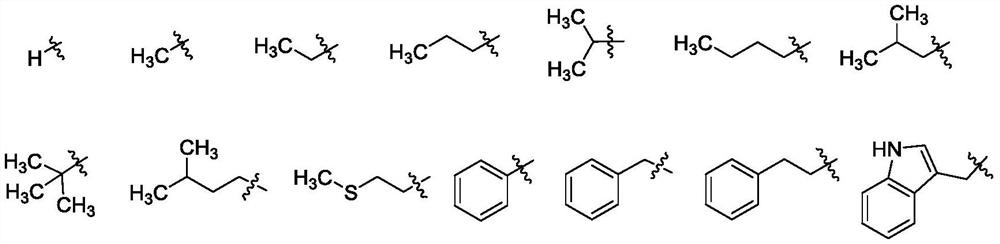
L-amino acid-6-gliotoxin ester trifluoroacetate and preparation method thereof
A technology for ester trifluoroacetate and gliotoxin, which is applied in the field of L-amino acid-6-gliotoxin ester trifluoroacetate compound and its preparation, can solve poor stability, large toxic and side effects, synthesis and Weak research on structure-activity relationship, etc., to achieve the effect of expanding structure types and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] Weigh 200mg of gliotoxin and dissolve it in 4mL of dichloromethane, stir at room temperature and add 1.1eq of N-Boc-L-sarcosine, 1eq of DCC and 0.1eq of DMAP, and perform TLC monitoring every 30min during the reaction , After 1.5-2h, the reaction is complete. Add 50mL of dichloromethane to the reaction system for dilution, and dilute the reaction system with saturated NH 4 The Cl solution was washed three times, and the aqueous phase was extracted once with dichloromethane. The organic phases were combined, washed three times with saturated NaCl solution, dried over anhydrous magnesium sulfate for 12 h, the organic phase was concentrated to obtain a crude extract, which was separated and purified by column chromatography to obtain a light yellow solid. Dissolve the above product in dichloromethane, add 300 μL trifluoroacetic acid under ice bath and stir, monitor by TLC every 30 minutes during the reaction, after 2-3 hours the reaction is complete, remove th...
Embodiment 2
[0031]
[0032] N-Boc-L-sarcosine was replaced by N-Boc-methyl-L-alanine, and other operations were the same as in Example 1 to obtain light yellow solid 3b with a yield of 65%, m.p.: 125.9-126.8°C; IR (KBr,cm -1 ):3429,1761,1685,1420,1383,1202,1062,798,722,703,672. 1 H NMR (400MHz, DMSO-d 6 )δ9.33(s,2H),6.06(dt,J=5.8,3.1Hz,1H),5.98(ddd,J=8.1,4.8,2.7Hz,1H),5.65(d,J=9.8Hz,1H ),5.18(d,J=12.7Hz,1H),5.08(d,J=12.7Hz,1H),4.86(d,J=13.1Hz,1H),4.56(d,J=13.1Hz,1H), 4.25(q,J=7.1Hz,1H),3.23(s,1H),3.18(s,1H),3.10(s,3H),2.62(s,3H),1.67(ddq,J=39.4,8.2, 3.9Hz, 1H), 1.48(d, J=7.2Hz, 3H). 13 C NMR (100MHz, DMSO-d 6 )δ168.17, 164.92, 163.06, 158.13, 132.21, 129.42, 123.66, 119.08, 118.09, 75.79, 75.32, 72.63, 69.45, 61.04, 54.92, 35.71, 30.43, 28.03, 13.63 17 h 22 N 3 o 5 S 2 [M-CF 3 COO - ] + :412.0995, found 412.1100.
Embodiment 3
[0034]
[0035] N-Boc-L-sarcosine was replaced by N-Boc-L-2-aminobutyric acid, and other operations were the same as in Example 1 to obtain light yellow solid 3c with a yield of 48%, m.p.: 121.6-122.5°C; IR( KBr,cm -1 ):3433,2928,1762,1685,1414,1379,1356,1203,1137,1061,835,799,722. 1 H NMR (400MHz, DMSO-d 6 )δ8.52(s,3H),5.98(dd,J=5.1,2.8Hz,1H),5.90(ddd,J=9.7,5.0,2.8Hz,1H),5.58(d,J=9.8Hz,1H ),5.41(d,J=18.3Hz,1H),5.12(d,J=12.8Hz,1H),4.99(d,J=12.7Hz,1H),4.79(d,J=13.1Hz,1H), 4.49(dd,J=13.0,2.8Hz,1H),3.61-3.54(m,1H),3.16(d,J=1.7Hz,1H),3.11(d,J=1.6Hz,1H),3.03(s ,3H),1.84–1.76(m,2H),0.91(t,J=7.5Hz,3H). 13 C NMR (100MHz, DMSO-d 6 )δ168.32, 164.91, 163.05, 158.12, 132.27, 129.46, 123.63, 119.04, 114.97, 75.81, 75.31, 72.62, 69.48, 61.01, 52.89, 35.73, c28.04, 23.33, 8.9ld.ESI 17 h 22 N 3 o 5 S 2 [M-CF 3 COO - ] + :412.0995, found 412.1001.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com