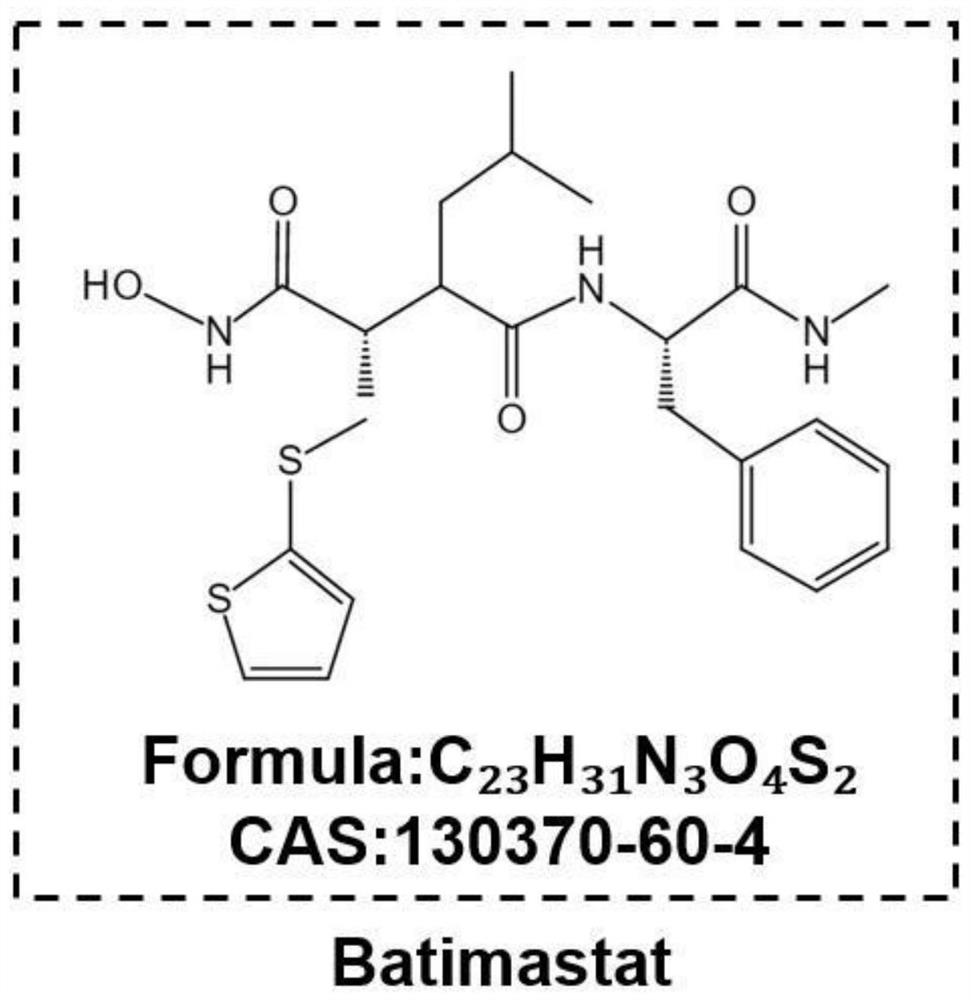
Application of Baatimatat to preparation of medicine for preventing and treating obesity and secondary bone loss
A technology for bone loss and obesity, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
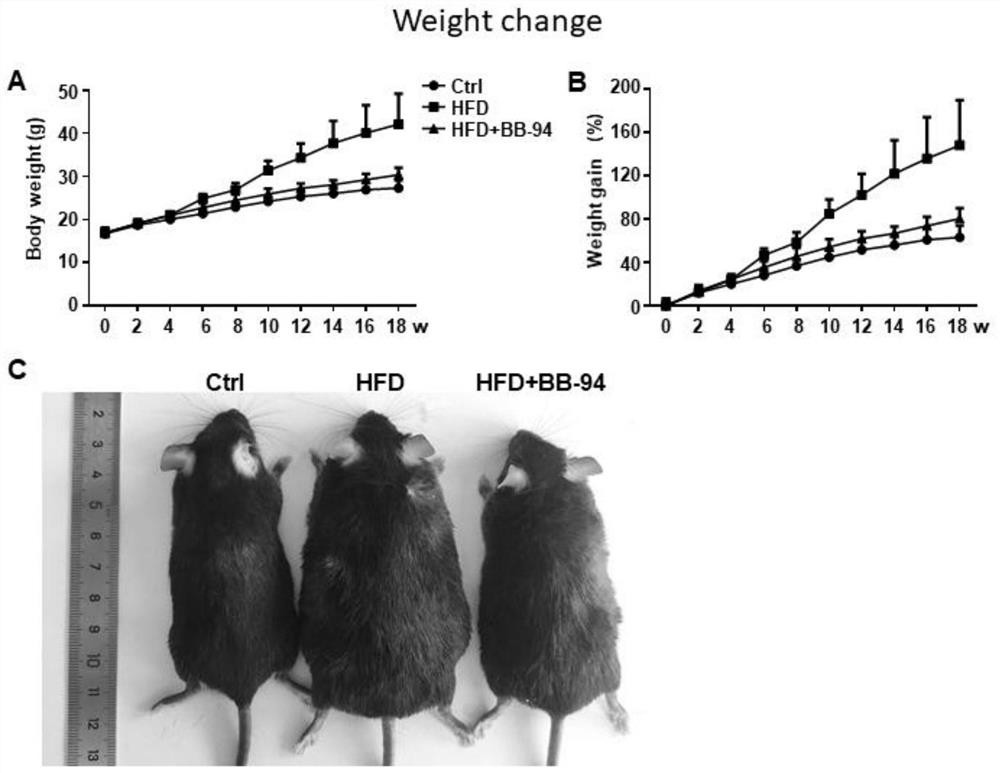
[0032] Example 1: Batimastat relieves obesity caused by high-fat diet
[0033] (1) Establishment of high-fat diet obesity model
[0034] Twenty-four 6-week-old male C57BL / 6 mice were randomly divided into 3 groups, 8 in each group, as control diet group (Ctrl), high-fat diet group (HFD) and high-fat diet BB-94 intervention group (HFD +BB-94). Among them, the control group was fed with purified 10% fat for energy, and the high-fat diet group and high-fat diet BB-94 intervention group were fed with purified 60% fat for energy with high-fat diet. After starting to replace the purified feed for modeling, the mice in the high-fat diet BB-94 intervention group were intraperitoneally injected with BB-94 every 2 days (using the pre-prepared 6 mg / mL Batimastat solution for intraperitoneal injection, 50 μL / 10 g for injection, so that the final The dosage is 30 mg / kg), and the mice in the control group and the high-fat diet group are injected intraperitoneally with the same amount of c...
Embodiment 2
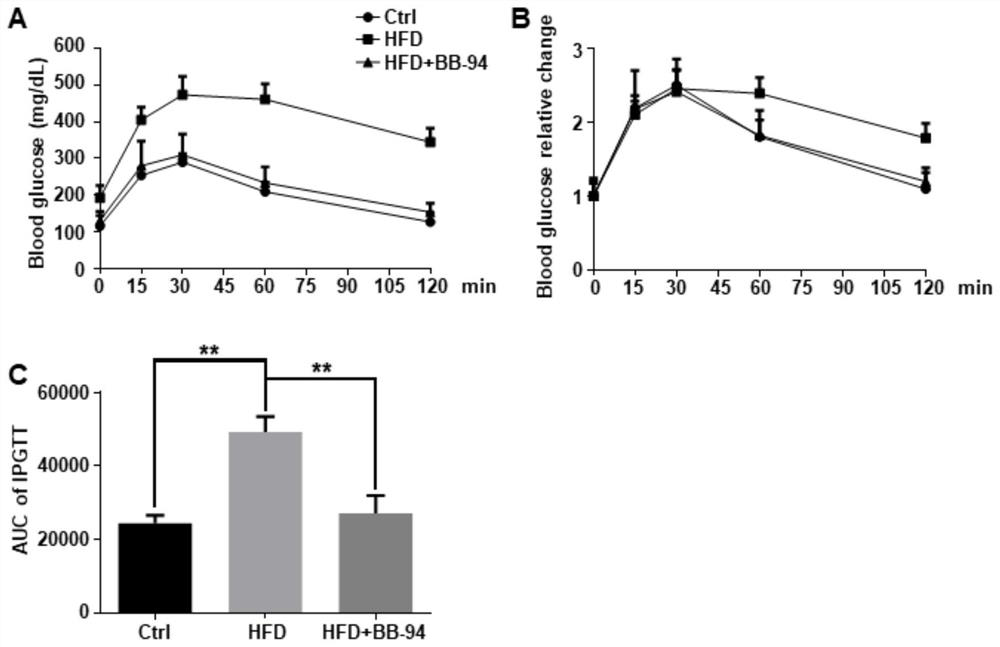
[0037] Example 2: Batimastat alleviates glucose metabolism disorder caused by high-fat diet
[0038] (1) Batimastat relieves impaired glucose tolerance in mice fed a high-fat diet ( image 3 )
[0039] In order to study the effect of BB-94 on the glucose tolerance of obese mice induced by high-fat diet, we used intraperitoneal glucose tolerance test (IPGTT) at the 16th week after modeling to evaluate the mice after HFD modeling. Changes in glucose tolerance and the effect of BB-94 intervention. At the 16th week after the high-fat diet obesity model was established, the mice in each group were fasted for 8 hours (removing food and keeping drinking water) for intraperitoneal glucose tolerance test. D-(+)-glucose was dissolved in sterilized PBS to prepare a 0.1 g / mL glucose solution, which was injected intraperitoneally at a dose of 100 μL / 10 g (the final dose was 1 g / kg). Collect tail vein blood at 0, 15, 30, 60, and 120 minutes respectively, using Roche The Active blood gl...
Embodiment 3
[0044] Example 3: Effect of Batimastat on white adipose tissue in various parts of obese mice with high-fat diet
[0045] (1) Batimastat alleviated the accumulation of white adipose tissue in the abdominal cavity of mice fed a high-fat diet ( Figure 5 )
[0046] On the basis of the previous study on the effects of BB-94 on body weight, glucose metabolism disorder, and insulin resistance in obese mice fed a high-fat diet, we further evaluated its effect on white adipose tissue in various parts of obese mice fed a high-fat diet by gross morphology and histological staining. influences. First of all, when the mice were collected, a large amount of white fat accumulation (epididymal white adipose tissue) could be observed in the abdominal cavity of the mice in the HFD group after opening the abdominal cavity, while this situation was significantly improved in the mice in the HFD+BB-94 group.
[0047] (2) Batimastat alleviated the hypertrophy and hypertrophy of white adipose tis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com