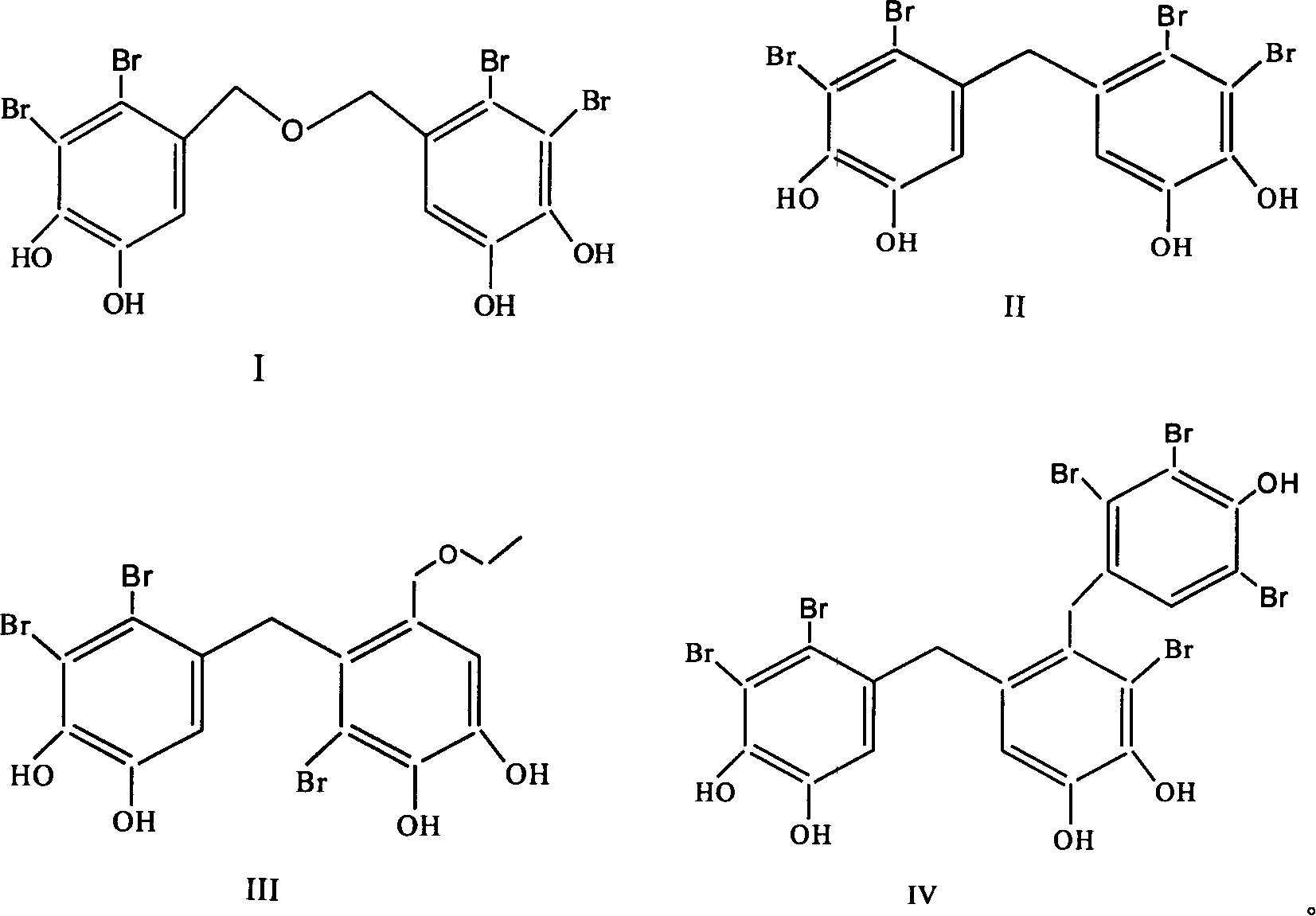
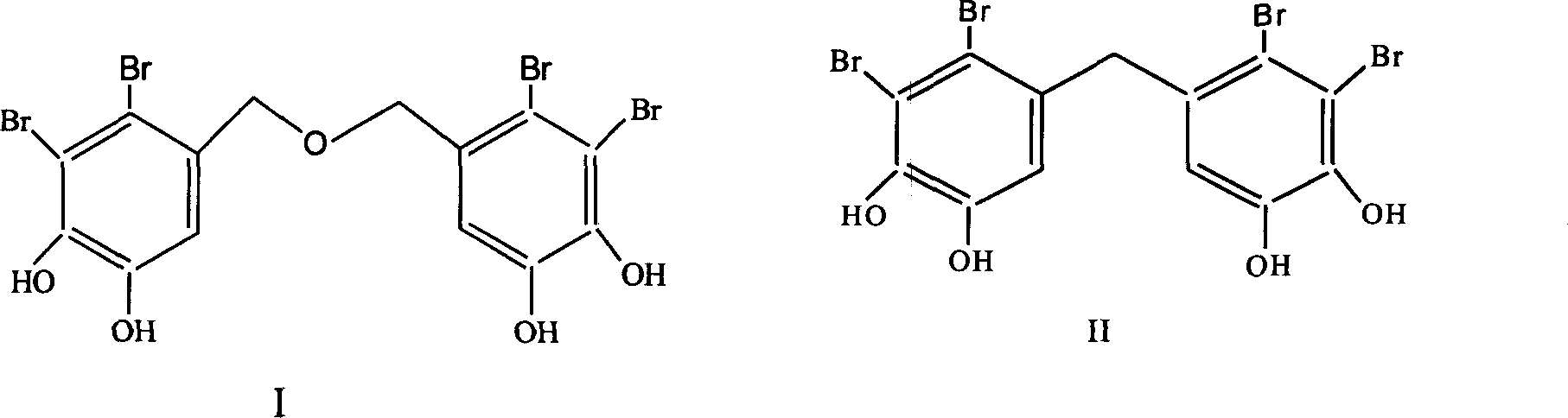
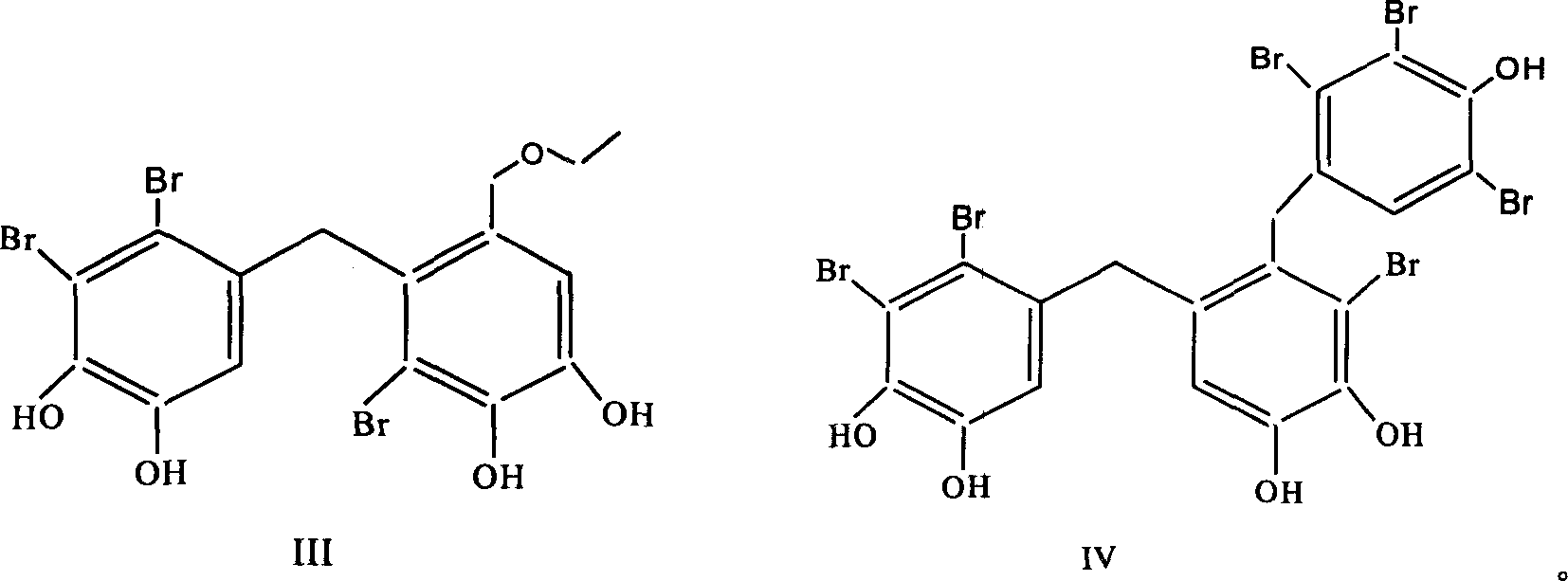
Use of bromphenol compound in protein-tyrosine phosphonatease inhibitor
A tyrosine phosphatase and compound technology, applied in the field of bromophenol compounds, can solve problems such as few research reports on the chemical composition of seaweed, and achieve the effect of treating and preventing diabetes and obesity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the extraction of bromophenol compound
[0023] Get 15 kg of pine knotweed after air-drying, soak and extract 3 times with 95% ethanol at room temperature, each time for 72 hours; Ethyl acetate extraction. Ethyl acetate extracts were subjected to silica gel column chromatography, first with petroleum ether-acetone (100:0~1:1) and chloroform-methanol (100:0~0~100) gradient elution, and thin layer chromatography to check the same fraction . Chloroform:methanol 5:1 eluted fraction, chloroform:methanol 3:1 eluted fraction, petroleum ether:acetone 2:1 eluted fraction, petroleum ether:acetone 1:1 eluted fraction were subjected to repeated silica gel column chromatography to obtain the compound I (1082 mg), II (1171 mg), III (2644 mg), IV (776 mg).
Embodiment 2
[0024] Embodiment 2: Bromophenol compounds inhibit protein tyrosine phosphatase activity test in vitro:
[0025] PTP1B: GST fusion protein expressed and purified in Escherichia coli;
[0026] Substrate: PNPP
[0027] Positive reference substance: sodium orthovanadate (2μM)
[0028] Test principle: The product obtained by hydrolyzing the phospholipid of the substrate PNPP by PTP1B has a strong light absorption at 410nm, so the change of absorption at 410nm can be directly detected to observe the change of enzyme activity and the inhibitory effect of the compound on it.
[0029] Test method: Take the above-mentioned 4 kinds of bromophenol compounds, and prepare 2 solutions of 200 μM and 20 μM of test products with phosphate buffer solution respectively.
[0030] Sample serial number
[0031] The test results show that the sample is significantly higher than the traditional protein tyrosine phosphatase inhibitor orthovanadate in inhibiting the activity of protein tyros...
Embodiment 3
[0032] Example 3 In vivo hypoglycemic test of the total bromophenol compounds of Pinus chinensis
[0033] Animals: 40 Kunming white mice.
[0034] Model: mouse diabetes model induced by streptozotocin
[0035] The specific operation is as follows:
[0036] Take 40 Kunming mice weighing 20-24g, and divide them into normal control group, test product 1 (4.0 g / kg), test product 2 (2.0 g / kg) group. The normal control group was intraperitoneally injected with normal saline, and the rest of the groups were intraperitoneally injected with streptozotocin (130 mg / kg). After 48 hours, blood was collected from the orbital vein of the mice, and the serum was separated to measure the serum glucose level. Then the administration group was intragastrically administered once a day, and the normal control group and the model group were given equal volumes of distilled water for 10 consecutive days. After fasting for 16 hours on the 11th day, blood was collected from the orbital venous plexu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com