Methods of treating immunotherapy-related toxicity using a gm-csf antagonist
A GM-CSF, immunotherapy technology, used in the use of GM-CSF antagonists to treat immunotherapy-related toxicity, can solve problems such as unknown long-term effects and prolonged hospitalization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0304] Preparation of IV.hGM-CSF Antibody
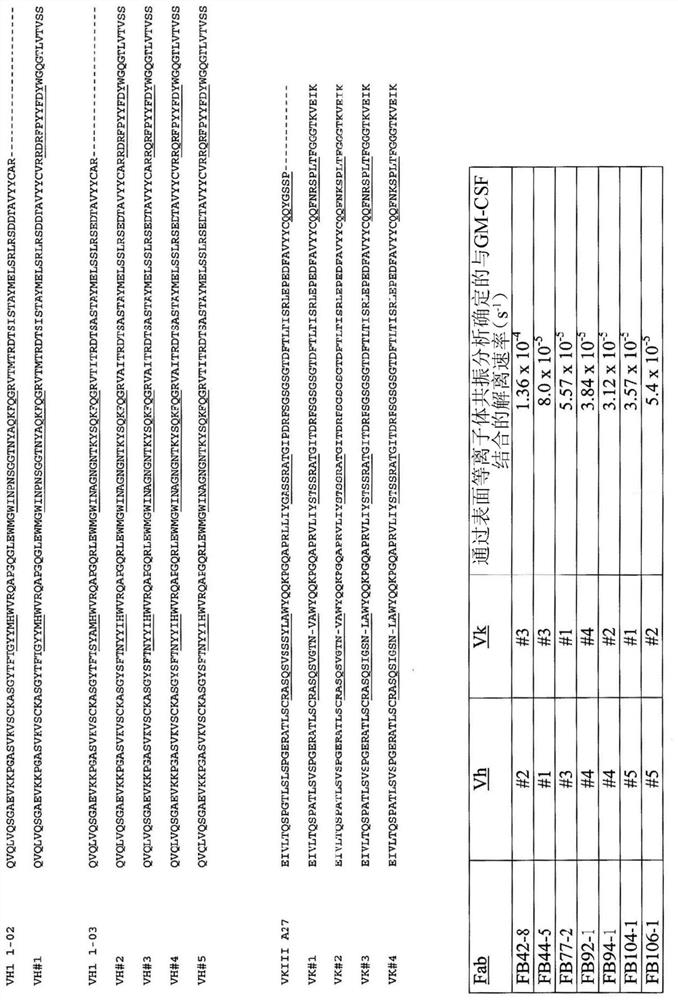
[0305] Antibodies of the present invention may include such as figure 1 V shown in H Any one of areas VH#1, VH#2, VH#3, VH#4, or VH#5. In some embodiments, antibodies of the invention may include figure 1 V shown in L Any one of areas VK#1, VK#2, VK#3 or VK#4. In some embodiments, the antibody has figure 1 V shown in H zone VH#1, VH#2, VH#3, VH#4 or VH#5; and if figure 1 V shown in L Region VK#1, VK#2, VK#3 or VK#4, as described, for example, in US Patent Nos. 8,168,183 and 9,017,674, each of which is incorporated herein by reference in its entirety.
[0306] Antibodies can be tested to confirm that the antibodies retain activity to antagonize hGM-CSF activity. Antagonist activity can be determined using any number of endpoints, including proliferation assays. Neutralizing antibodies and other hGM-CSF antagonists can be identified or evaluated using a number of assays that assess hGM-CSF function. For example, cell-based h...
example 1
[0357] Example 1 - Exemplary Humanized Antibodies to GM-CSF
[0358] A panel of engineered Fab' molecules with cl9 / 2 specificity was generated from an epitope-focused human V segment library as described in US Patent Application Publication Nos. 20060134098 and 20050255552. The epitope-focused library was constructed from human V segment library sequences joined to the CDR3-FR4 region containing the BSD sequence in CDRH3 and CDRL3 and the human germline J segment sequence. For the heavy chain a human germline JH4 sequence was used and for the light chain a human germline JK4 sequence was used.
[0359] Full-length human engineered V regions that support binding to recombinant human GM-CSF were selected from a Vhl-restricted library. As described in US Patent Application Publication No. 20060134098, a "full-length" V-κ library was used as the basis for constructing a "cassette" library in which only part of the murine cl9 / 2 V segment was initially replaced by a library of huma...
example 2
[0366] Example 2—Evaluation of Human Engineered GM-CSF Antibodies
[0367] This example was evaluated in a cell-based assay compared to a chimeric IgGlk antibody (Ab2) with variable regions from mouse antibody LMM102 (Nice et al., Growth Factors 3:159, 1990) The binding activity and biological efficacy of the human engineered anti-GM-CSF antibody were investigated. Ab1 is a human engineered IgG1k antibody against GM-CSF with the same constant region as Ab2.
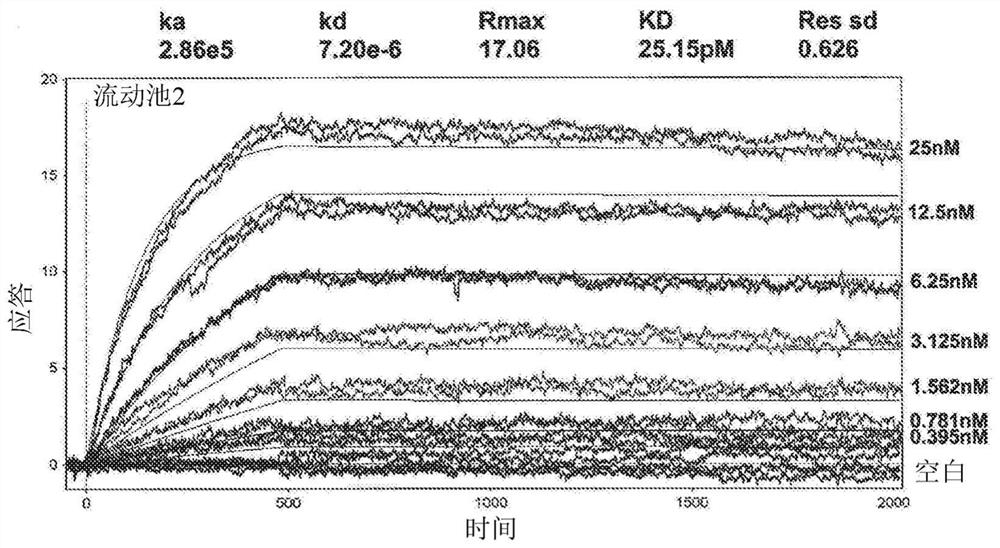
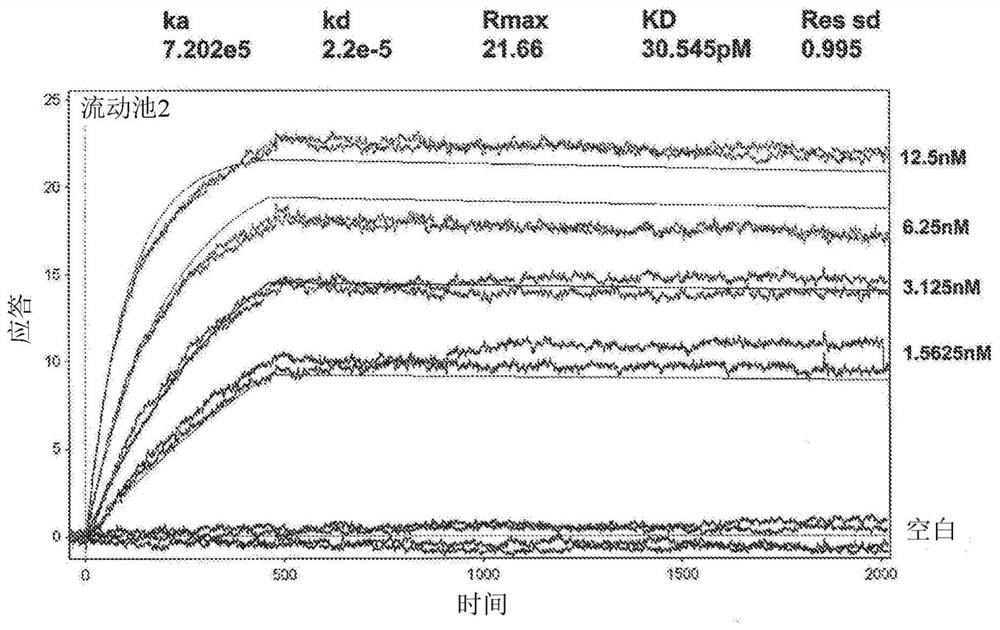
[0368] Surface Plasmon Resonance Analysis of Binding of Human GM-CSF to Ab1 and Ab2
[0369] Surface plasmon resonance analysis was used to compare the binding kinetics and monovalent affinities of the interaction of Ab1 and Ab2 with glycosylated human GM-CSF using a Biacore 3000 instrument. Ab1 or Ab2 was captured onto the Biacore chip surface using polyclonal anti-human F(ab')2. Glycosylated recombinant human GM-CSF expressed from human 293 cells was used as analyte. Kinetic constants were determined in 2 independen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com