Antibodies directed to filamin-a and therapeutic uses thereof
A filamin and antibody technology, applied in the direction of antibodies, immunoglobulins, hybrid immunoglobulins, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0313] Example 1: Preparation of Human Chimeric Filamin-A Antibody
[0314] AHO1402 (a monoclonal mouse filamin-A IgGIk antibody) was obtained from Life Technologies as a solution in 95% BSA and 5% antibody. To remove BSA and purify antibodies, antibodies were separated and purified by gel electrophoresis, separated by size, and excised from the gel.
[0315] Gel slices containing antibodies were de novo sequenced using Database Assisted Shotgun Sequencing (DASS) technology, which utilizes high-resolution MS / MS peptide determination to obtain sequence information, followed by overlapping peptide sequence analysis. Compile to reconstruct the sequence. The DASS method is generally described on the Creative Biolabs website (http: / / www.creative-biolabs.com / next-generation-antibody-sequencing.html).
[0316] The constructed amino acid sequences of the light and heavy chain variable domains were then added to the murine and human constant region sequences chosen by the inventors. ...
Embodiment 2
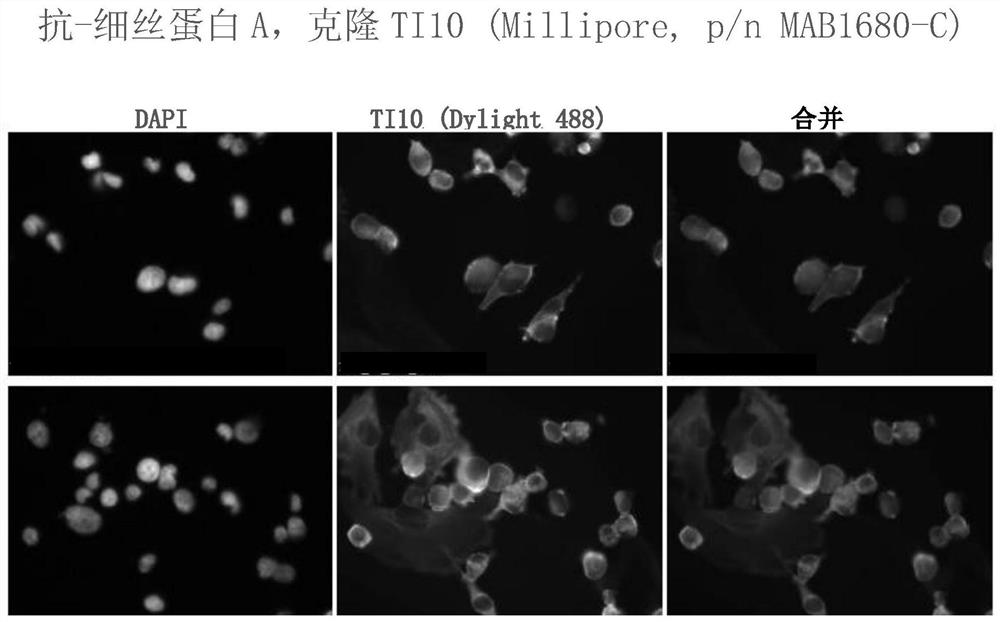
[0318] Example 2: scFv preparation and characterization
[0319] The heavy and light chain variable regions were subcloned into scFv vector systems to generate scFv in two possible orientations, light-heavy (VL-VH or vl-vh) and heavy-light (VH-VL or vh- vl).
[0320] The resulting single chain antibodies will be tested in assays including immunofluorescence staining of cells, cell motility, proliferation, apoptosis and other assays to assess functional biological activity.
[0321] The scFvs will be further evaluated for use in diagnostic assays and methods of treating diseases such as breast cancer.
Embodiment 3
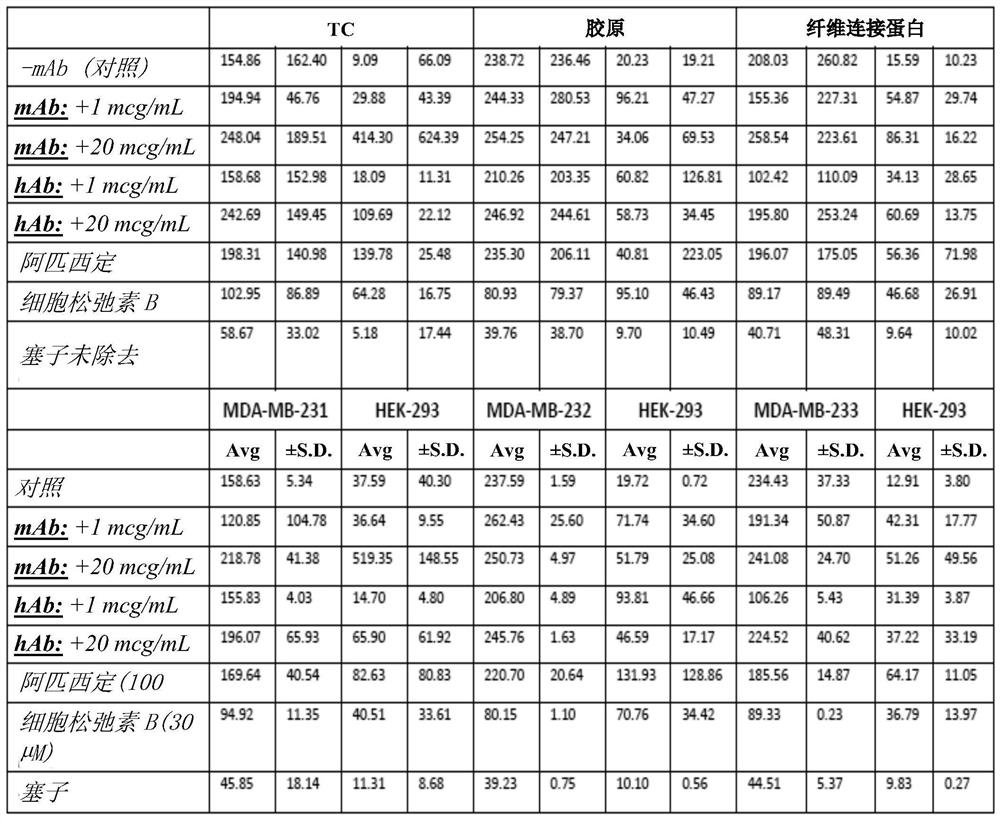
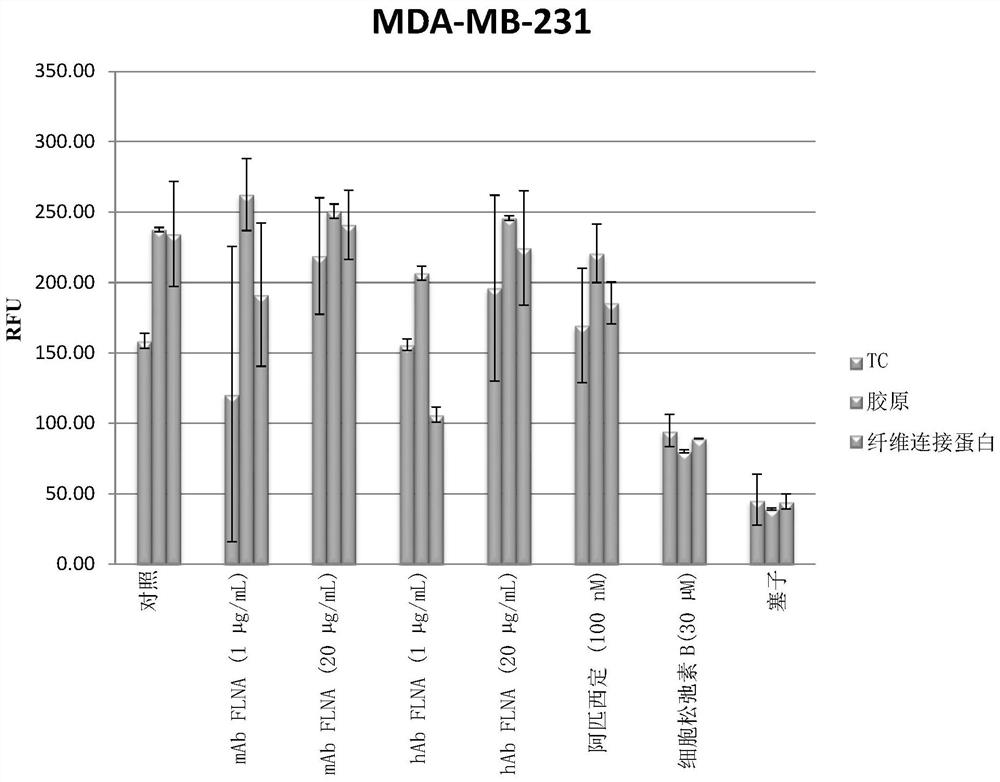
[0322] Example 3: Determination of cell motility
[0323] Cell motility assays were used to explore the functional activity of the mAbs generated in this disclosure (mouse antibody B186, human chimeric antibody B185 and human chimeric antibody B411).
[0324] Evidence of activity was obtained with murine and human chimeric antibodies. Surprisingly, however, human chimeric mAbs showed greater biological activity than murine mAbs. The effect was also more pronounced on fibronectin-coated tissue culture surfaces compared to collagen-coated surfaces.
[0325] Cell motility assay utilizing Platypus Technologies ORIS TM Cell migration assay platform, which is generally described at http: / / www.platypustech.com / discoverAssay.html.
[0326] This measure of motility, including 3D iterations, is thought to correlate with cancer cell invasiveness and malignancy, and is therefore a surrogate for cancer metastasis and other viability.
[0327] Specifically, in ORIS TM Filamin-A antibod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com