Anti-erythropoietin receptor peptide
A halogen atom and prodrug technology, applied in the field of anti-erythropoietin receptor peptides and compositions containing the peptides, can solve problems such as unclear details, achieve strong EpoR inhibitory ability, high cancer cell killing ability, improve Effects of absorption/distribution/metabolism/excretion kinetics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0418] (Example 1: Synthesis of peptides)
[0419] The following peptides were synthesized (the names of the compounds are indicated in parentheses):
[0420] Ac-SCHFGPLTWVCK-NH 2 (YS12) [SEQ ID NO: 9]
[0421] (Benzoyl)-SCHFGPLTWVCK-NH 2 (GT11255)
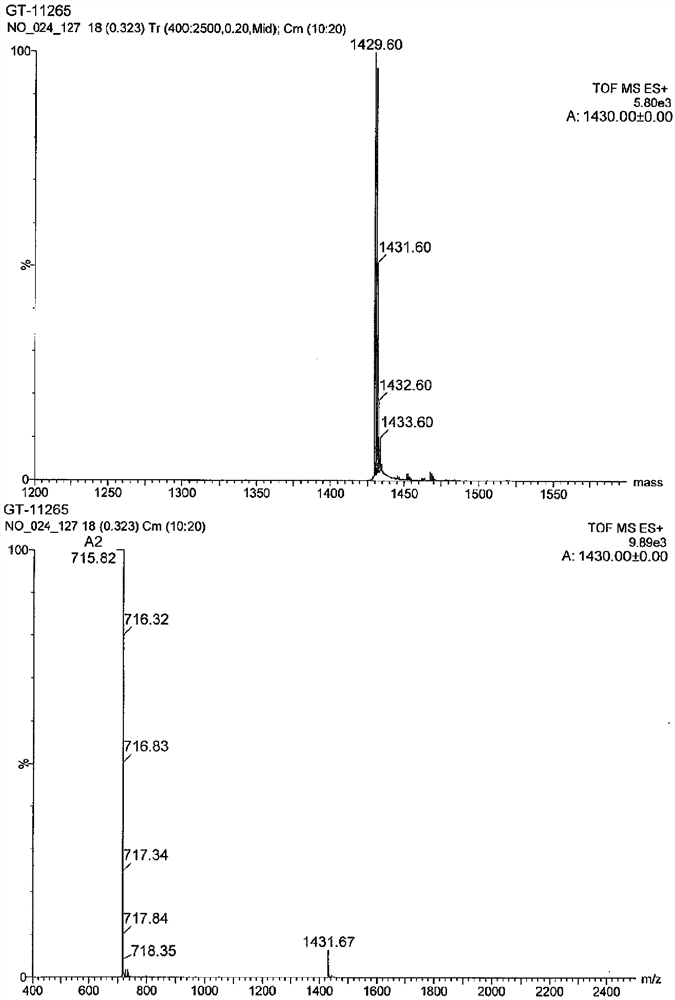
[0422] (p-Fluorophenylacetyl)-SCHFGPLTWVCK-NH 2 (GT-11256)
[0423] (Propionyl)-SCHFGPLTWVCK-NH 2 (GT-11257)
[0424] Ac-SCH(p-fluoro-Phe)GPLTWVCK-NH 2 (GT-11258)
[0425] Ac-SCH(p-chloro-Phe)GPLTWVCK-NH 2 (GT-11259)
[0426] Ac-SCH(m-chloro-Phe)GPLTWVCK-NH 2 (GT-11260)
[0427] Ac-SCHYGPLTWVCK-NH 2 (GT11261) [SEQ ID NO: 10]
[0428] Ac-SCH(Phenylglycine)GPLTWVCK-NH 2 (GT-11262)
[0429] Ac-SCH(Phenylethylglycine)GPLTWVCK-NH 2 (GT-11263)
[0430] Ac-SCHFAPLTWVCK-NH 2 (GT-11264) [SEQ ID NO: 11]
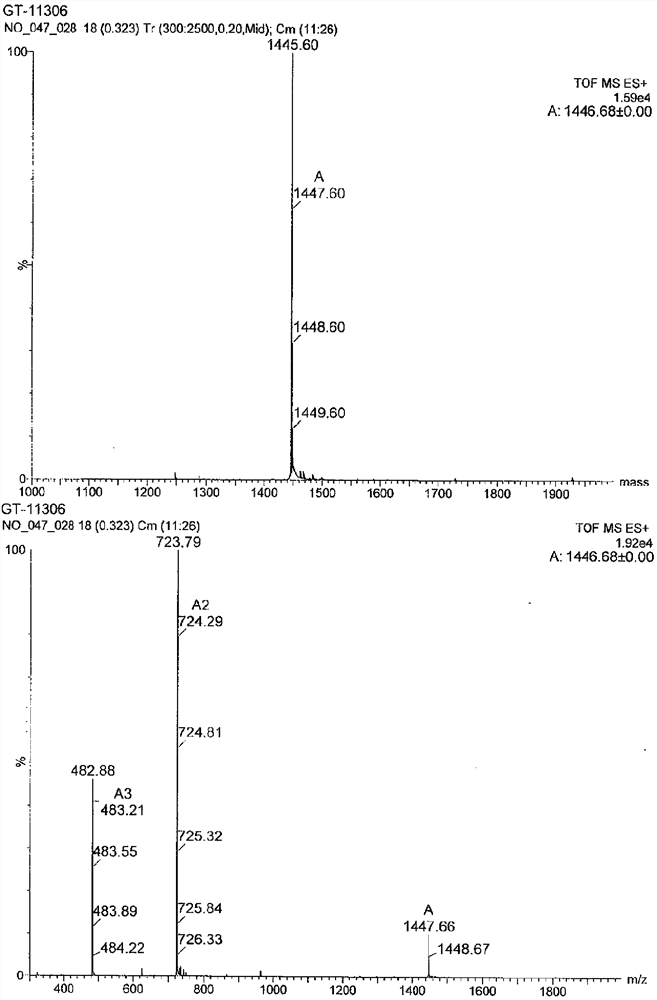
[0431] Ac-SCHFaPLTWVCK-NH 2 (GT-11265)
[0432] Ac-SCHFGALTWVCK-NH 2 (GT-11266) [SEQ ID NO: 12]
[0433] Ac-SCHFG (Homoproline) LTWVCK-NH 2 (GT-11267)
[0434] Ac-SCHFGPATWVCK-NH 2 (GT-11268) [SEQ ID NO: 13]
[...
Embodiment 2
[0457] (Example 2: In vitro performance evaluation in human liver cancer cells)
[0458] The properties of the synthetic peptides were measured by the following methods.
[0459] Lethal effect based on synthetic peptides using HepG2 cells derived from human liver cancer
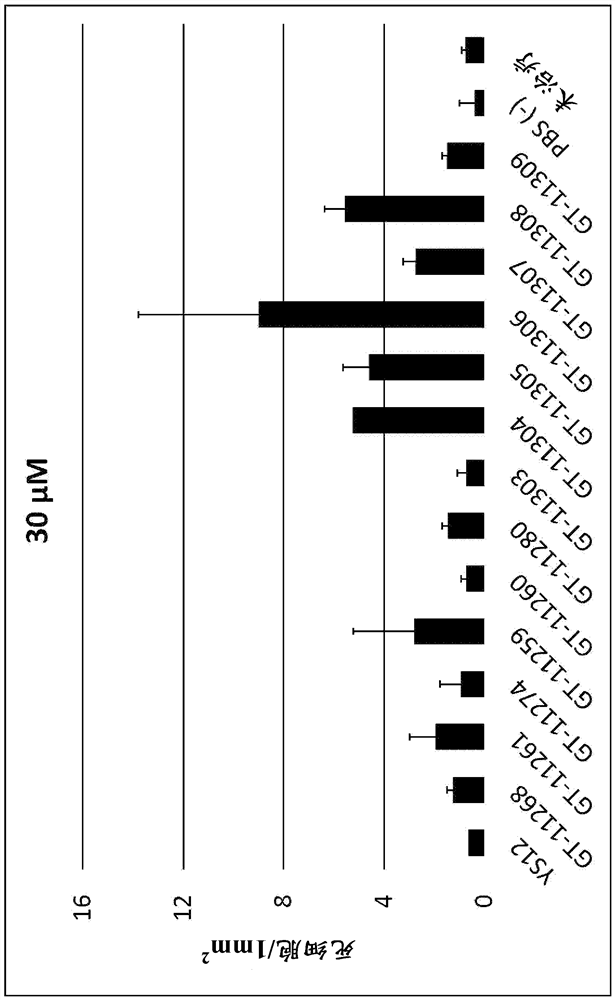
[0460] Tested against the following test compounds; YS12, GT-11268, GT-11261, GT-11274, GT-11259, GT-11260, GT-11280, GT-11303, GT-11304, GT-11305, GT-11306 , GT-11307, GT-11308, and GT-11309.
[0461] HepG2 cells (ATCC, HB-8065) were seeded on 8-well CHAMBER SLIDE (Nunc)), and the lethal effect was studied at 4 concentrations of the test compound.
[0462]Culture conditions: use D-MEM medium containing 10% fetal bovine serum (Fujifilm Wako Pure Chemical Industries, 045-30285), at 37°C, 5% CO 2 Cultured under -95% air condition.
[0463] Test concentration; 120 mu M, 60 mu M, 30 mu M and 15 mu M was the test compound, and PBS(-) was used as a control.
[0464] Cell number; 1.0~1.5×10 3 The num...
Embodiment 3
[0475] (Example 3: In vitro performance evaluation of human pancreatic cancer cells)
[0476] The in vitro performance of the synthetic peptides on human pancreatic cancer cells was evaluated by the following method.
[0477] AsPC-1 cells (ECACC, 96020930) from human pancreatic cancer were used in RPMI-1640 medium (Sigma , R8758-500ML), at 37°C, 5% CO 2 Subculture was carried out under -95% air condition (twice / week). Use 0.05% trypsin-0.02% EDTA solution (Sigma, T4174-100ML) to recover the AsPC-1 cells from the culture dish, suspend them in the above medium, and inoculate them in 96-well microplates (3×10 3 / 0.05mL / well). After culturing overnight, add test compounds (total 43 compounds, final concentration 150 mu M) medium (0.05 mL / well, 3 wells / test compound). After 72 hours, the cell proliferation ability of each well was measured by the WST method (Dojin Chemical Research Institute, Cell Counting Kit-8), and the inhibition rate of each test compound relative to the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com