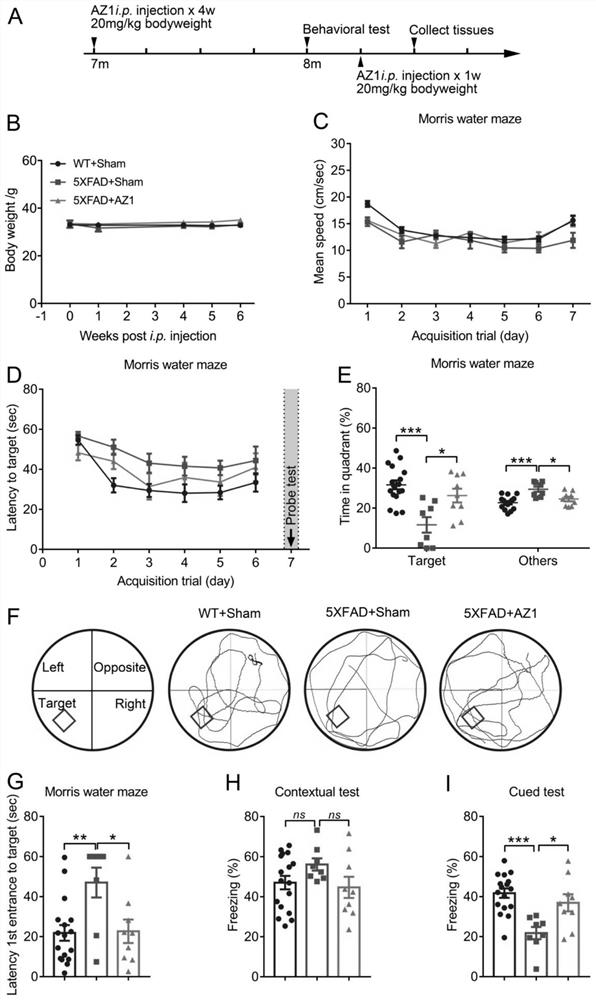
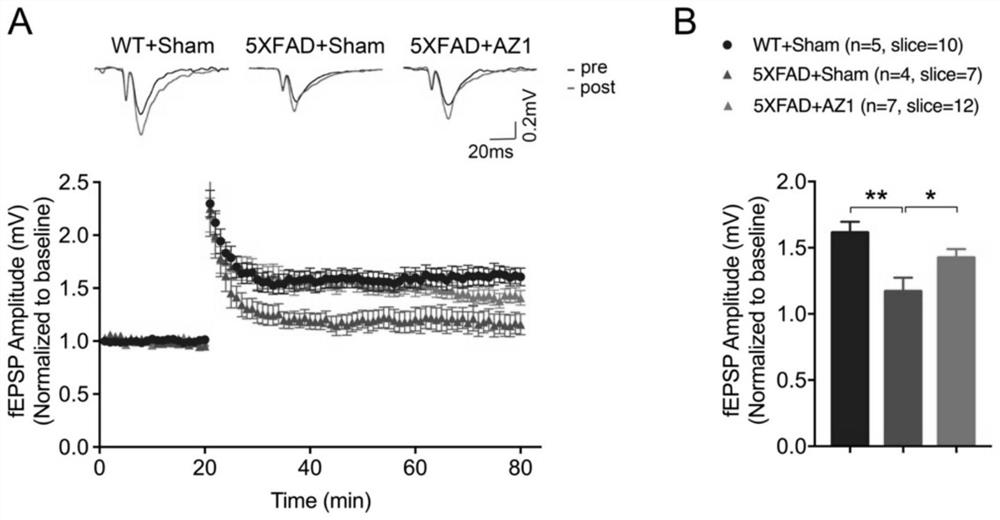
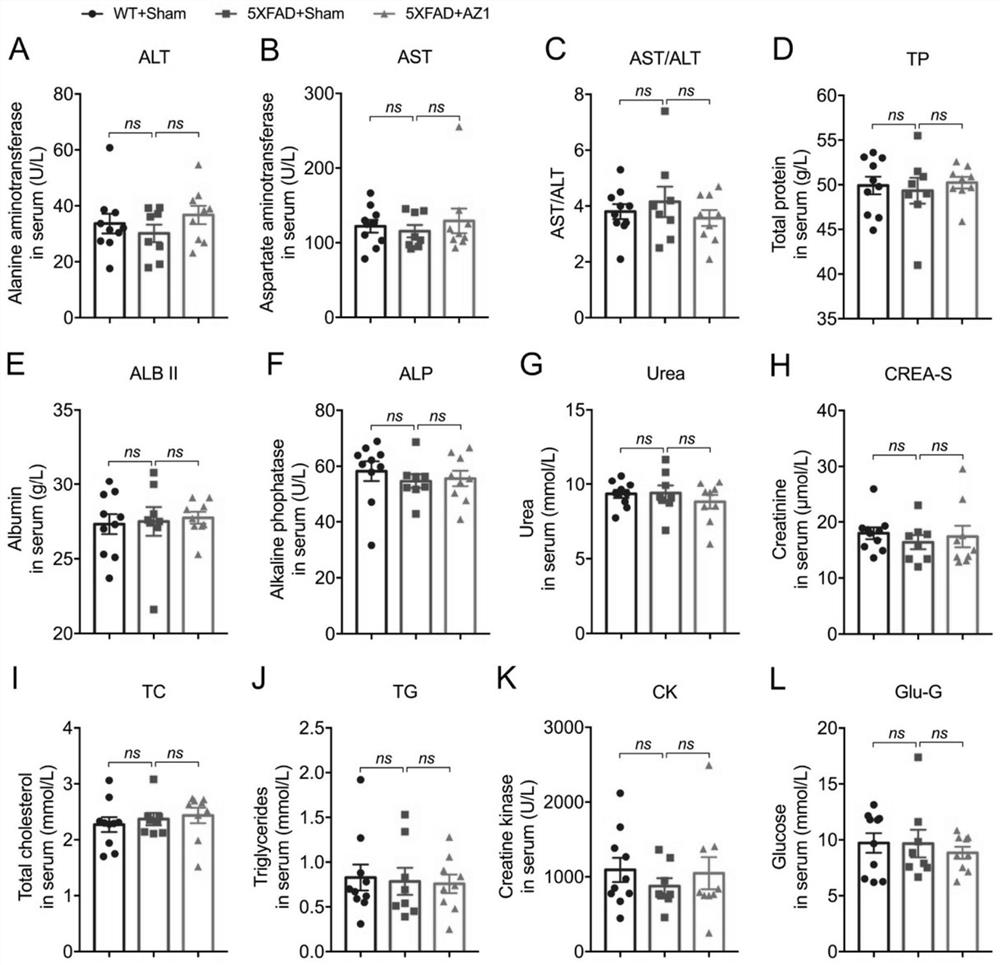
Compound for treating alzheimer's disease
A technology for Alzheimer's disease, compounds, applied in the field of medicine, which can solve the problems of lack of treatment, prevention or mitigation of Alzheimer's disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1. Preparation of compound XMU1
[0128]
[0129] Step 1) Under nitrogen protection, potassium carbonate (2.93g, 21.22mmol), 4-(bromomethyl)-1-fluoro-2-(trifluoromethyl)benzene (3g, 11.67mmol) and 5-bromo -2-Hydroxybenzaldehyde (2.13g, 10.61mmol) was successively added to a 250ml three-neck flask, then 60ml DMF was added, and magnetically stirred at room temperature 25°C to obtain a suspension, which was stirred overnight at room temperature (about 15 hours), and TLC the next day Monitor the disappearance of starting material and terminate the reaction. The reaction solution was poured into 1.2L ice water, there was a white precipitate, the white solid obtained was filtered, washed with 3×500ml water, and dried in vacuo to obtain 5-bromo-2-(4-fluoro-3-(trifluoromethyl)benzyl oxy)benzaldehyde (3.5 g, 87.46%) as a white solid. The following reaction formula is shown:
[0130]
[0131] Step 2) Under nitrogen protection, add 5-bromo-2-(4-fluoro-3-(trifluoro...
Embodiment 2
[0134] Embodiment 2. Preparation of compound (2)
[0135]
[0136] Step 1) 3,5-bistrifluoromethylbenzyl bromide (0.22mL, 1.2mmol), 4-difluoromethoxy-3-hydroxybenzaldehyde (0.13g, 1mol), potassium carbonate (0.41g, 3mmol ), DMF (10 mL) were successively added into a 100 ml single-necked round bottom flask, reacted at room temperature for 6 h, extracted with ethyl acetate, combined the organic phases, concentrated under reduced pressure, and then purified on a silica gel column to obtain a white solid (0.37 g, 89.3%).
[0137] 1 H NMR (400MHz, CDCl 3 )δ9.95(s,1H),7.94(s,2H),7.88(s,1H),7.58(d,J=1.8Hz,1H),7.55(dd,J=8.1,1.8Hz,1H), 7.39(d, J=8.1Hz, 1H), 6.65(t, J=73.2Hz, 1H), 5.29(s, 2H).
[0138] MS(ESI):m / z 415.05[M+H] + ;
[0139] Step 2) 4-difluoromethoxy-3-(3,5-bistrifluoromethylbenzyl)benzaldehyde (0.21g, 0.5mmol), ethanolamine (0.09ml, 1.5mmol), acetic acid (0.17mL , 1.5mmol), THF (10mL) were sequentially added into a 100mL three-necked round-bottomed flask, protecte...
Embodiment 3
[0142] Embodiment 3. Preparation of compound (5)
[0143]
[0144] Step 1) 3,5-bistrifluoromethylbenzyl bromide (0.22mL, 1.2mmol), 4-difluoromethoxy-3-hydroxybenzaldehyde (0.13g, 1mol), potassium carbonate (0.41g, 3mmol ), DMF (10 mL) were successively added to a 100 ml single-necked round bottom flask, reacted at room temperature for 6 h, extracted with ethyl acetate, combined the organic phases, concentrated under reduced pressure, and then purified on a silica gel column to obtain a white solid (0.37 g, 89.3%).
[0145] 1 H NMR (400MHz, CDCl 3 )δ9.95(s,1H),7.94(s,2H),7.88(s,1H),7.58(d,J=1.8Hz,1H),7.55(dd,J=8.1,1.8Hz,1H), 7.39(d, J=8.1Hz, 1H), 6.65(t, J=73.2Hz, 1H), 5.29(s, 2H).
[0146] MS(ESI):m / z 415.05[M+H] + ;
[0147] Step 2) 4-difluoromethoxy-3-(3,5-bistrifluoromethylbenzyl)benzaldehyde (0.21g, 0.5mmol), N-methylpiperazine (0.17mL, 1.5mL) , acetic acid (0.17mL, 1.5mmol), THF (10mL) were sequentially added to a 100mL three-necked round-bottomed flask, protecte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com