Benzo-hexatomic ring derivative used as DPP-4 inhibitor and application of benzo-hexatomic ring derivative
A solvate, heterocycle technology, applied in the field of medicinal chemistry, can solve the problem of insufficient insulin secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
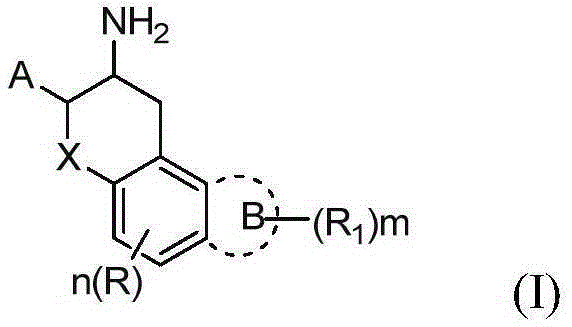
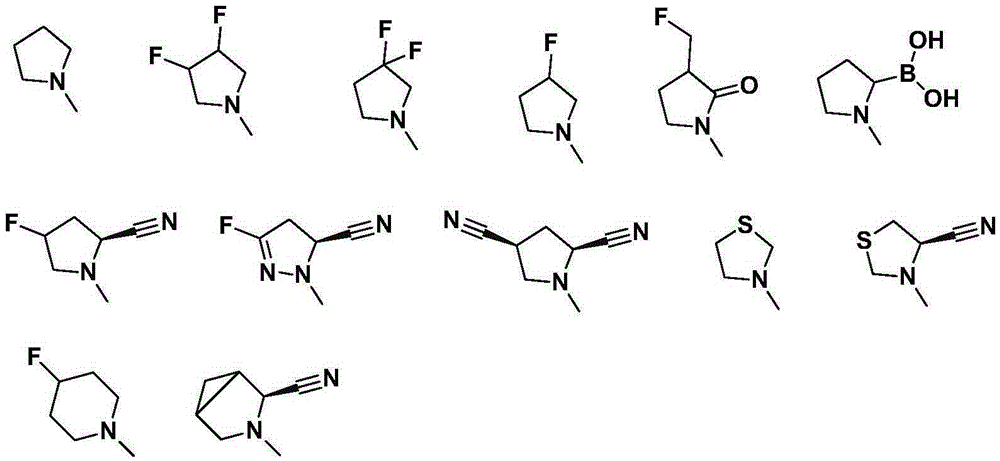
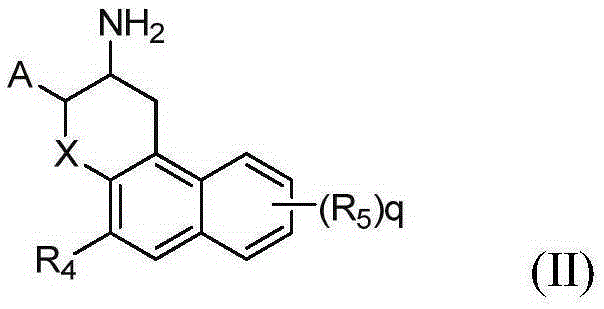
[0129] 1. trans-(2RS,3RS)-3-amino-2-(2,4,5-trifluorophenyl)chroman-5-ol (compound-1)
[0130]
[0131] Synthesis of Intermediate 1-1:
[0132] 2,4,5-Trifluorobenzaldehyde (10g, 62.46mmol), nitromethane (4mL), methanol (10mL) were prepared into a solution; methanol (60mL), water (30mL), sodium hydroxide (2.5N , 30mL) into a solution, keeping the temperature at 5°C; adding the former solution dropwise to the latter solution for about 30-60 minutes, and keeping the solution temperature at 5-10°C; Add the above solution dropwise into a mixed solution of zinc chloride (42.6g, 31.25mmol), concentrated hydrochloric acid (13mL), and water (17mL), keep the temperature at 0-10°C during the dropwise addition, and react at room temperature for 2-4h after the dropwise addition; After that, vacuum filtration was performed, and the filter cake was washed several times with 40% methanol solution to obtain 9.8 g of the product with a yield of 77%. GC-MS: 203.
[0133] 1 H-NMR (400MHz, C...
Embodiment 31
[0521] Example 31. DPP4 Inhibitory Activity of Compounds of the Invention
[0522] The inventors have detected the inhibitory activity of the compounds of the present invention to DPP4 at the enzyme level through the following experiments:
[0523] Enzyme level activity evaluation:
[0524] Name: DPP4 (dipeptidyl peptidase 4); alias: CD26; ADABP; ADCP2; DPPIV;
[0525] Screening method:
[0526] Method name: DPP4 activity assay, fluorescence.
[0527] Instrument: microplate reader, Envision (PerkinElmer, USA).
[0528] Material: Human DPP4, which was expressed in insect cells using baculovirus expression system in this experiment. The substrate is Gly-Pro-AMC.
[0529] process:
[0530] DPP4 can specifically hydrolyze the substrate Gly-Pro-AMC to generate the product AMC. AMC is excited by 355nm ultraviolet light to generate 460nm emission light. The linear change of fluorescence value at 460nm wavelength per unit time is dynamically measured, and the DPP4 activity is ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com