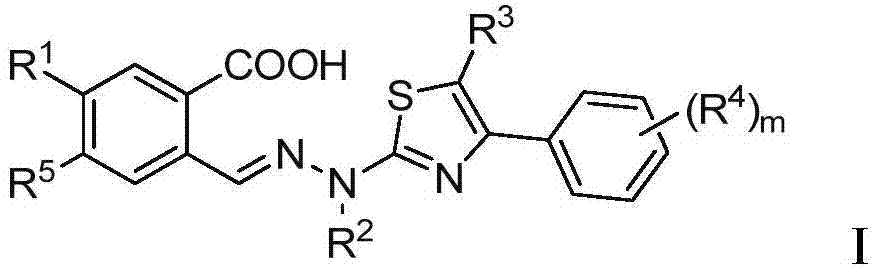
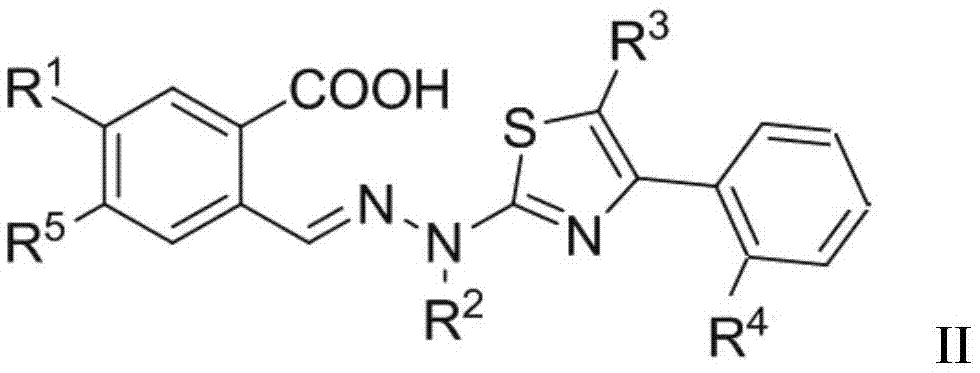
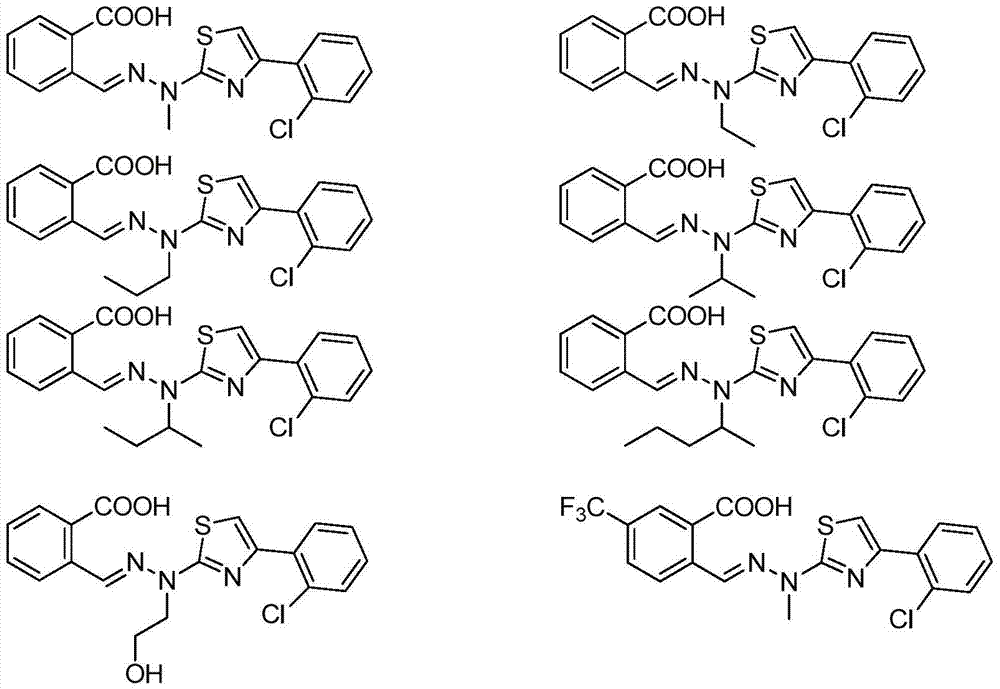
Thiazole derivative and application thereof in restraining dihydroorate dehydrogenase
A technology of drugs and compounds, applied in the application of diseases, the field of synthesis of thiazole derivatives, can solve the problems of toxic side effects, narrow therapeutic window of Brequinar, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] 2-Methylthiosemicarbazide(1)
[0080]
[0081] Weigh 2.5g (17.3mmol) of methylhydrazine sulfate in a 250ml single-necked bottle, add 100ml of ethanol, add 1.6g (20.8mmol) of ammonium thiocyanate under stirring, heat to reflux, and react for 72 hours, then cool the reaction solution to After suction filtration at room temperature, the filtrate was spin-dried by silica gel column chromatography (DCM / MeOH=40:1), and the second by-product was separated to obtain 0.63 g of a white powdery solid, with a yield of 34.2%. 1 H NMR (400MHz, DMSO-d 6 ,ppm)δ 7.36(s,2H),4.89(s,2H),3.41(s,3H).GC-MS(EI)calcd for C 2 h 7 N 3 S[M] + 105.0, found 105.0.
[0082] 2-Methyl-1-(2-carboxybenzyl)thiosemicarbazide (2)
[0083]
[0084] Weigh 80mg (0.76mmol) of compound (1) in a 50ml single-necked bottle, add 20ml of ethanol, add o-carboxybenzaldehyde 114mg (0.76mmol) under stirring, heat to reflux, monitor the reaction by TLC until the conversion of raw materials is complete, and coo...
Embodiment 2
[0153] Cultivation and purification of hsDHODH protein
[0154] Cultivation of protein: hsDHODH protein was obtained according to the hsDHODH gene sequence in GenBank according to conventional methods, such as conditions described in Sambrook et al., Molecular Cloning: A Laboratory Guide (NewYork: Cold Spring Harbor Laboratory Press, 1989).
[0155] Transform the recombinant plasmid pET-19b-DHODH with correct sequencing into E.coli BL21(DE3) competent, smear it on the LB plate containing ampicillin and culture it, and randomly pick the strains and inoculate it in the LB medium containing 100μM ampicillin Cultivate overnight at 37°C, 230rpm on a shaker. Inoculate at a ratio of 1:200 in 500 mL of LB medium containing 100 μL of ampicillin for expansion at 37 °C and 230 rpm. When the OD value of the bacteria reached 0.8-1, IPTG was added to the medium to make the final concentration of IPDG 0.5 mM, and the expression was induced overnight at 25°C. The induced bacteria were colle...
Embodiment 3
[0158]Enzyme level activity test of the compound represented by formula Ⅰ
[0159] The principle of enzyme level activity test is: firstly, dihydroorotate (DHO) is oxidatively dehydrogenated under the catalysis of DHODH to generate orotate (Orotate, OA), and at the same time, it is accompanied by the acceptance of flavin mononucleotide (FMN) 2H + and 2e - reduced to reduced flavin mononucleotide (FMNH 2 ); followed by coenzyme Q (C O Q) accept FMNH as hydrogen acceptor 2 electrons and protons are reduced to reduced coenzyme Q (C O QH 2 ), the reduced coenzyme Q transfers electrons to the chromogenic substrate dichloroindophenol sodium salt (DCIP), and finally DCIP is reduced. DCIP has a maximum absorption at 600nm, while the reduced DCIP has no absorption at 600nm. The degree of oxidation of the substrate DHO can be judged according to the weakening degree of absorbance. The degree of oxidation of the substrate DHO per unit time is the initial rate of the enzymatic rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com