A device for burning remaining blood for drug clinical trials
A technology for clinical trials and treatment devices, which is applied to the field of residual blood burning treatment devices for drug clinical trials, can solve the problems of poor sealing of blood collection tubes, exudation of residual blood, unpleasant odor, etc., so as to improve burning efficiency and effect. , to prevent the growth of bacteria, the effect of prolonging the residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
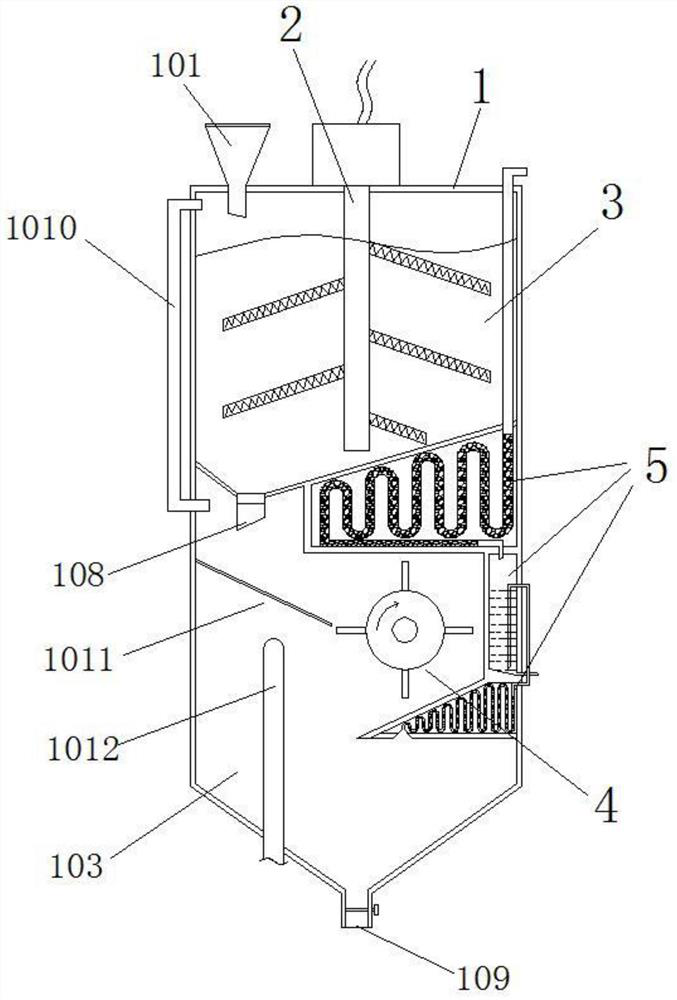
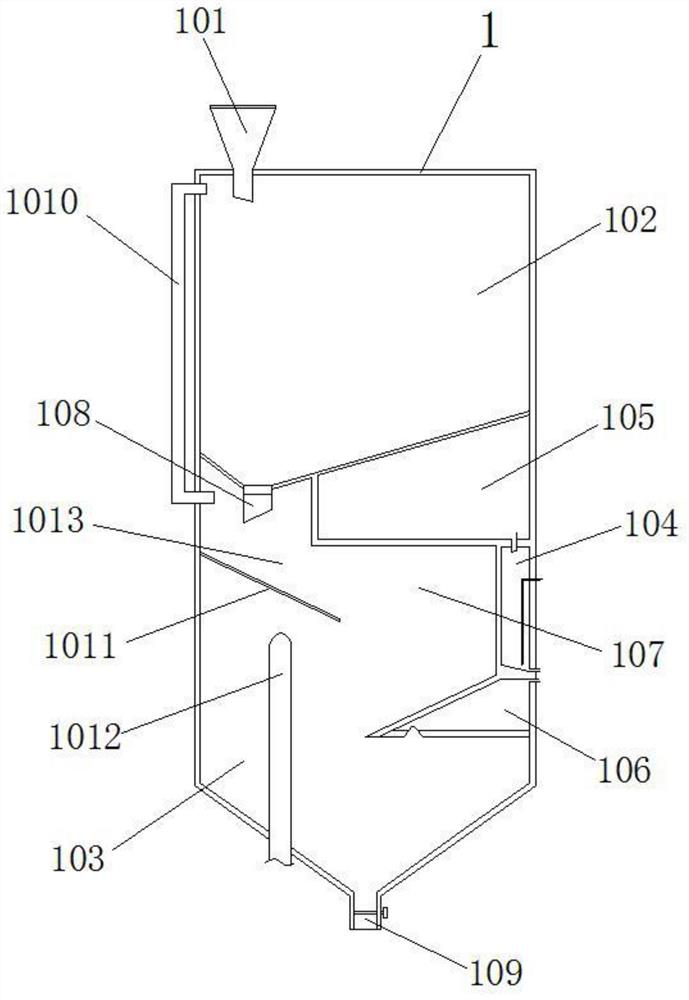
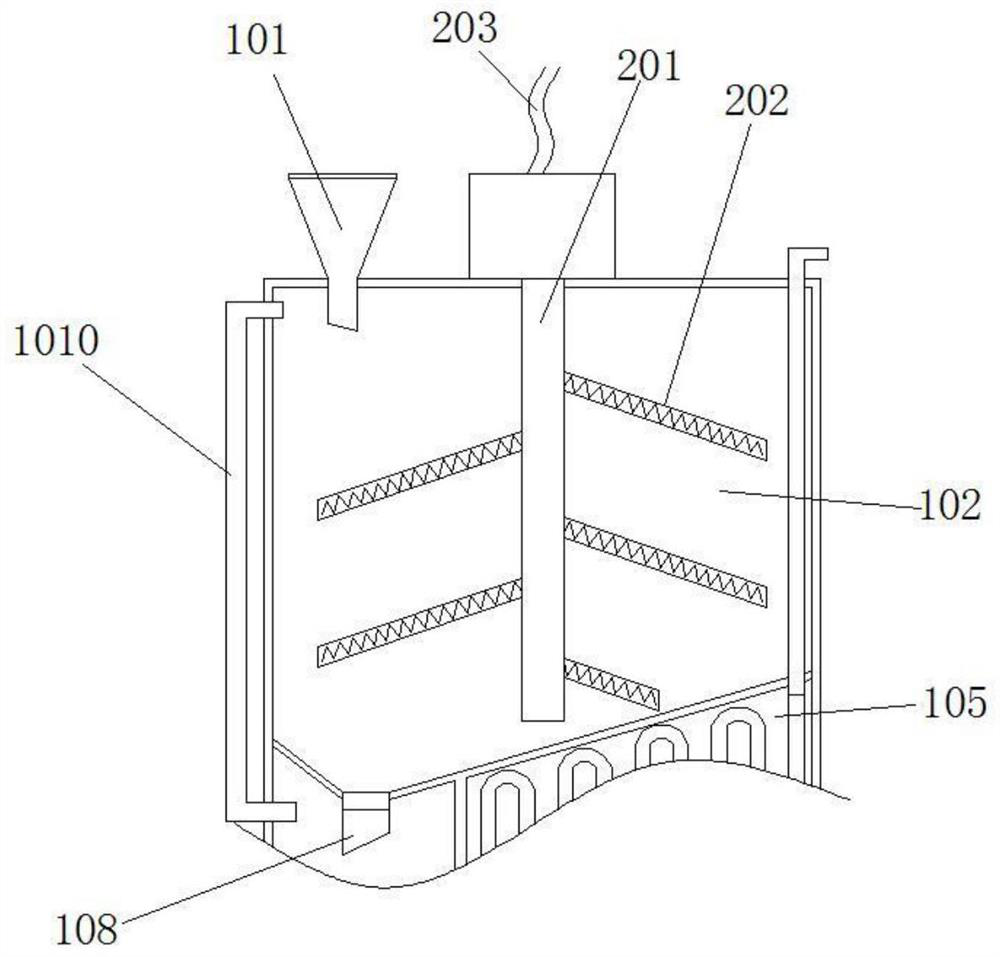
[0037] like figure 1 and 2 As shown in the figure, a residual blood burning treatment device for clinical trials of medicines includes a closed cylindrical case 1, and the cylindrical case 1 is divided into an upper residual blood adsorption chamber 102 and a lower burning treatment chamber 103, wherein, the residual blood adsorption cavity 102 is filled with the storage material 3 for adsorbing the residual blood, the top of the residual blood adsorption cavity 102 has a residual blood pouring port 101 into which residual blood is poured, and the residual blood pouring port 101 has a remnant blood pouring port 101. The open cover plate is inclined at the bottom, and a discharge port 108 for discharging the storage material to the burning treatment chamber 103 is provided at the lowest position. The discharge port 108 has a valve plate controlled by a solenoid valve, which is controlled by the PLC intelligent controller to open and Closed, a screen 1011 is inclined below the ...
Embodiment 2
[0041] This embodiment is an improvement on the basis of Embodiment 1, and its main structure is the same as that of Embodiment 1. The improvement lies in: the storage material 3 is iron sand, porous ceramic particles, montmorillonite particles and Quicklime is formed by mixing in a mass ratio of 2:3:4:1, and the particle sizes of iron sand, porous ceramic particles and montmorillonite particles are all 1-3mm.
Embodiment 3
[0043] This embodiment is another improvement scheme made on the basis of Embodiment 1, and its main structure is the same as that of Embodiment 1. The improvement points are as follows: Figure 7 As shown, the lifting and dispersing mechanism 4 includes a rotating drum 401 driven by the power mechanism to rotate. The axis direction of the rotating drum 401 is parallel to the side of the screen 1011 and is the same length as the side of the screen 1011; The outer wall of the rotating drum 401 is distributed with at least three metal elastic dials 402 having the same length as the rotating drum 401. The distance between the movement track of the metal elastic dials 402 and the side wall of the screen 1011 is 1 cm, and the rotating drum 401 drives the metal elastic dials 402. During the clockwise rotation, the particles with large particle size or high speed in the storage material rolled down along the screen 1011 are lifted up by the metal elastic plate 402 and returned to the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com