A preparation method and application of immobilized cells for tagatose production
A technology for immobilizing cells and tagatose, applied in the production and preparation of tagatose, and in the field of bioengineering, can solve the problems of uneven particle size of immobilized enzymes, loss of immobilization efficiency of enzymes, cumbersome production steps, etc., and achieve simple immobilization Chemical process, which is beneficial to separation and purification, and simplifies the effect of production and preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of whole cells of Bacillus subtilis
[0036] Pick and express thermostable α-glucan phosphorylase gene, express thermostable glucose phosphate mutase gene, express thermostable glucose phosphate isomerase gene, express thermostable 6-phosphate tagatose epimerase gene, Bacillus subtilis recombinant engineered bacteria expressing the heat-resistant 6-phosphate tagatose phosphatase gene (the starting bacterium is SCK6, see CN112342179B), and inoculated in LB medium respectively, and cultured overnight at 37°C with shaking. The culture was transferred to LB medium at an inoculum size of 1%, and cultured with shaking at 37°C overnight to obtain Bacillus subtilis fermentation broth expressing thermostable α-glucan phosphorylase and Bacillus subtilis expressing thermostable glucose phosphotransmutation Fermentation broth of Bacillus subtilis expressing thermostable glucose phosphate isomerase, fermentation broth of Bacillus subtilis expressing thermostab...
Embodiment 2
[0037] Embodiment 2: Production of tagatose by immobilized Bacillus subtilis
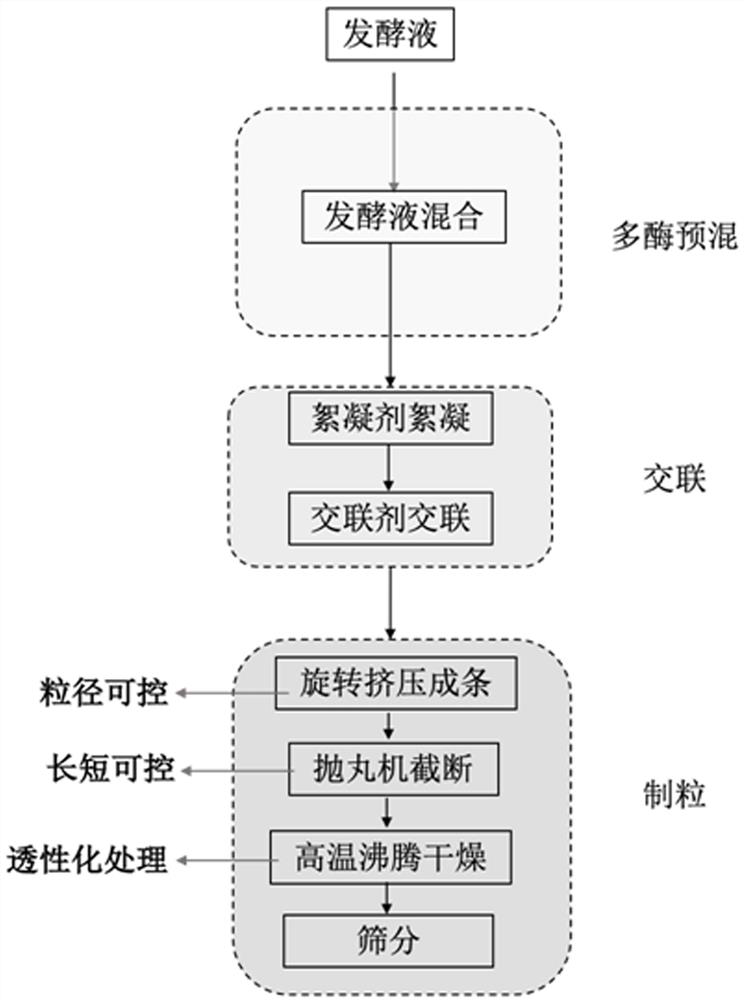
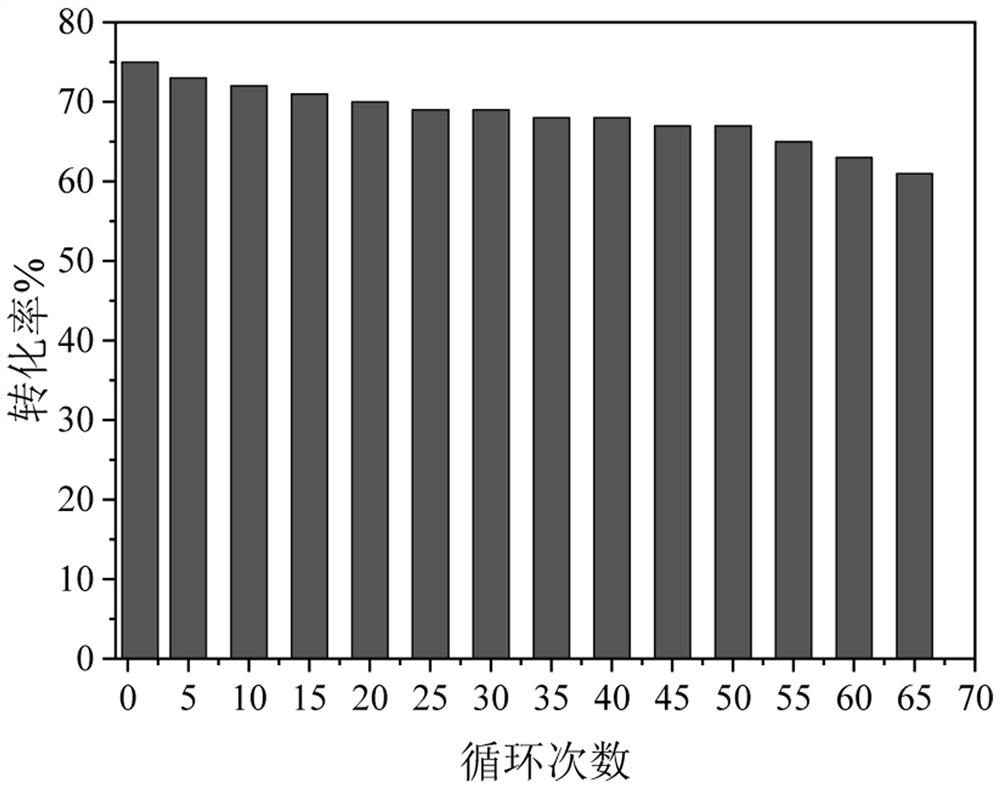
[0038] According to the OD600 ratio of 1:1:1:1:1, the Bacillus subtilis fermentation broth expressing thermostable α-glucan phosphorylase prepared in Example 1 and the Bacillus subtilis expressing thermostable glucose phosphomutase were fermented Bacillus subtilis fermentation broth expressing thermostable glucose phosphate isomerase, Bacillus subtilis fermentation broth expressing thermostable 6-phosphate tagatose epimerase, expressing thermostable 6-phosphate tagatose phosphatase The Bacillus subtilis fermentation broth was mixed so that OD 600 = 100, 1% w / v montmorillonite was added to the bacterial suspension, and stirred evenly. Subsequently, 0.5% w / v polyethyleneimine aqueous solution with a molecular weight of 10000 was added to flocculate at room temperature. Then, 2% v / v glutaraldehyde aqueous solution was added to crosslink for 2 h at room temperature. The filter cake is obtained after v...
Embodiment 3
[0040] Embodiment 3: Production of tagatose by immobilized Bacillus subtilis
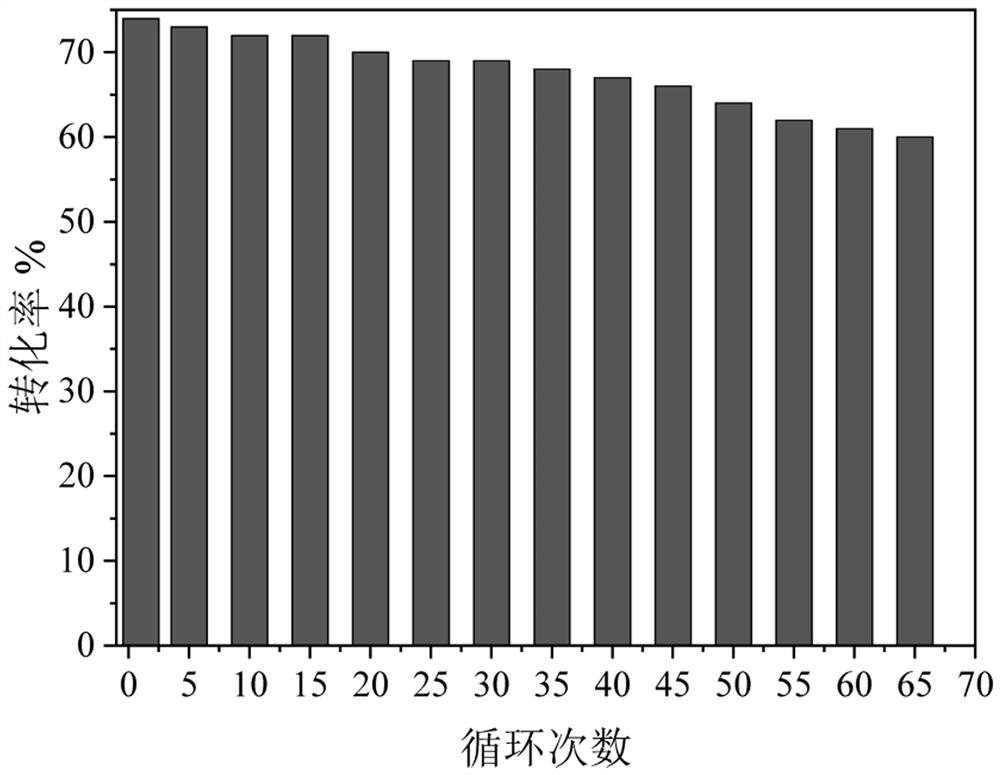
[0041] According to the OD600 ratio of 1:1:1:1:1, the Bacillus subtilis fermentation broth expressing thermostable α-glucan phosphorylase prepared in Example 1 and the Bacillus subtilis expressing thermostable glucose phosphomutase were fermented Bacillus subtilis fermentation broth expressing thermostable glucose phosphate isomerase, Bacillus subtilis fermentation broth expressing thermostable 6-phosphate tagatose epimerase, expressing thermostable 6-phosphate tagatose phosphatase The Bacillus subtilis fermentation broth was mixed so that OD 600 = 100, 5% w / v diatomaceous earth was added to the bacterial suspension, and stirred evenly. Subsequently, 0.1% w / v polyethyleneimine aqueous solution with a molecular weight of 70000 was added to flocculate at room temperature. Then, 1% v / v glutaraldehyde aqueous solution was added to crosslink for 2 h at room temperature. The filter cake is obtained afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com