Efficient red delayed fluorescent material and preparation method thereof
A delayed fluorescence and red technology, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problem of low efficiency of TADF small molecule red light materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
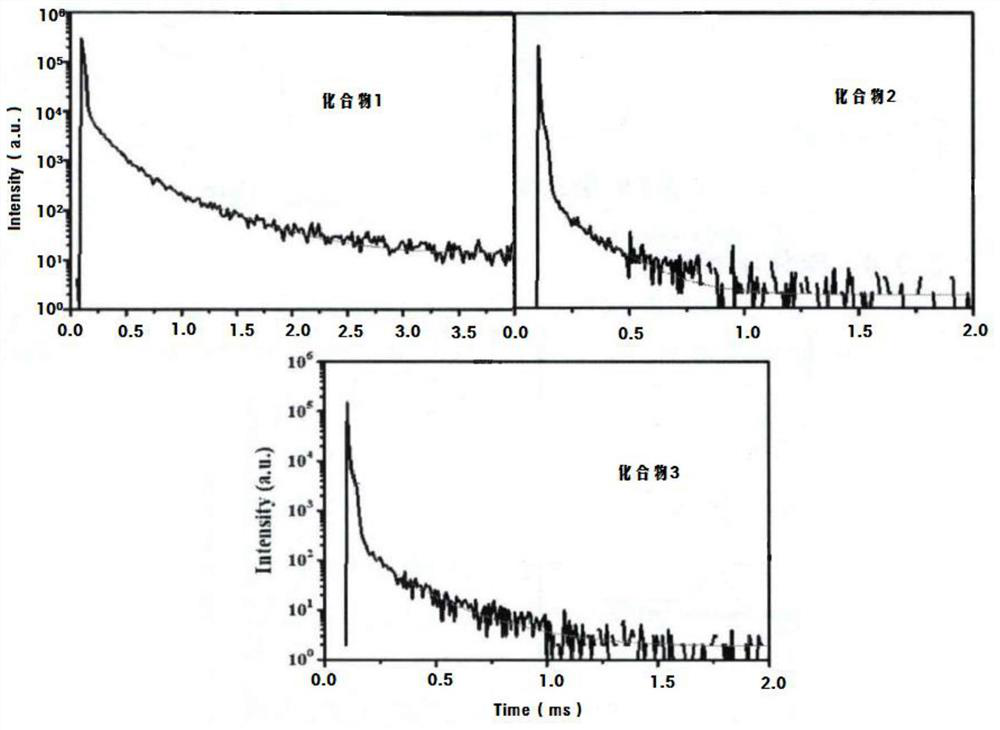
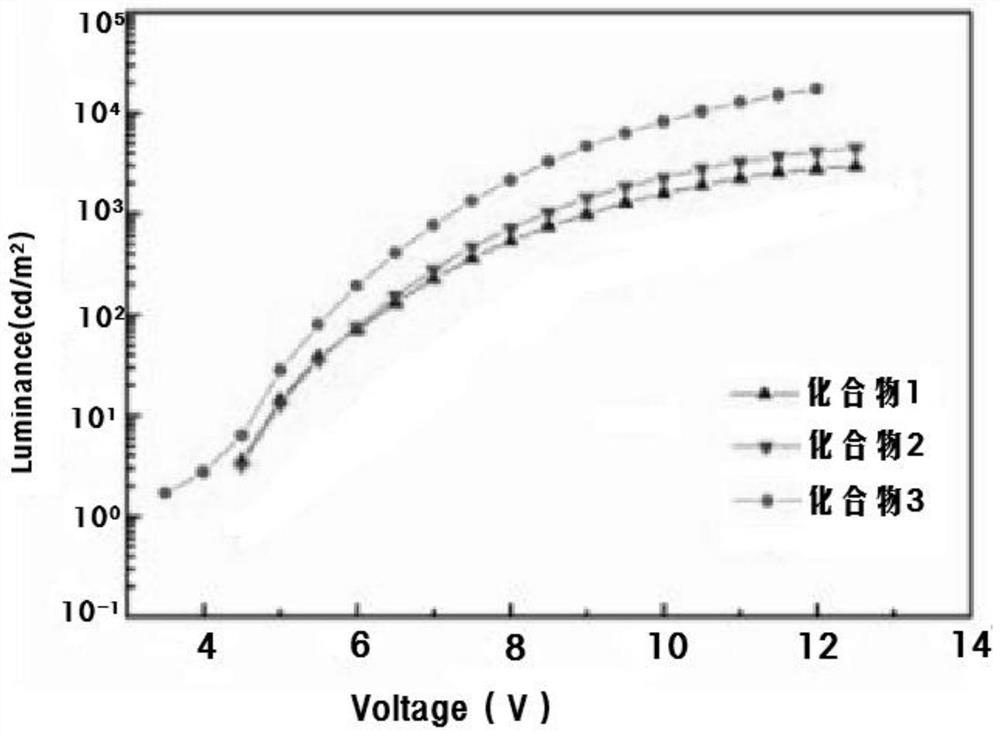
Image
Examples
Embodiment 1
[0022] Pyrene (2.02g, 10mmol) was dissolved in 50ml of hydrogen peroxide, and hydrogen bromide (1.21g, 15mmol), a mixed solution of ether and methanol (volume ratio=1:2) was added to the above solution, at 0°C After stirring for 8h, the obtained product was recrystallized in ethanol to obtain the product 1-bromopyrene (2.38g, yield 85%), and its chemical reaction equation was:
[0023]
Embodiment 2
[0025] 1-Bromopyrene (2.81g, 10mmol), isopropanol pinacol borate (3.46g, 20mmol), 100ml of anhydrous tetrahydrofuran and butyllithium (40mmol) were added to a 250ml three-necked flask, and argon was introduced After 10 minutes, the temperature was lowered to -78°C and the reaction was carried out for 18 hours. After the reaction was completed, extraction and purification were carried out to obtain the product 1-pyrene borate (2.33 g, yield 71%). The chemical reaction equation was:
[0026]
Embodiment 3
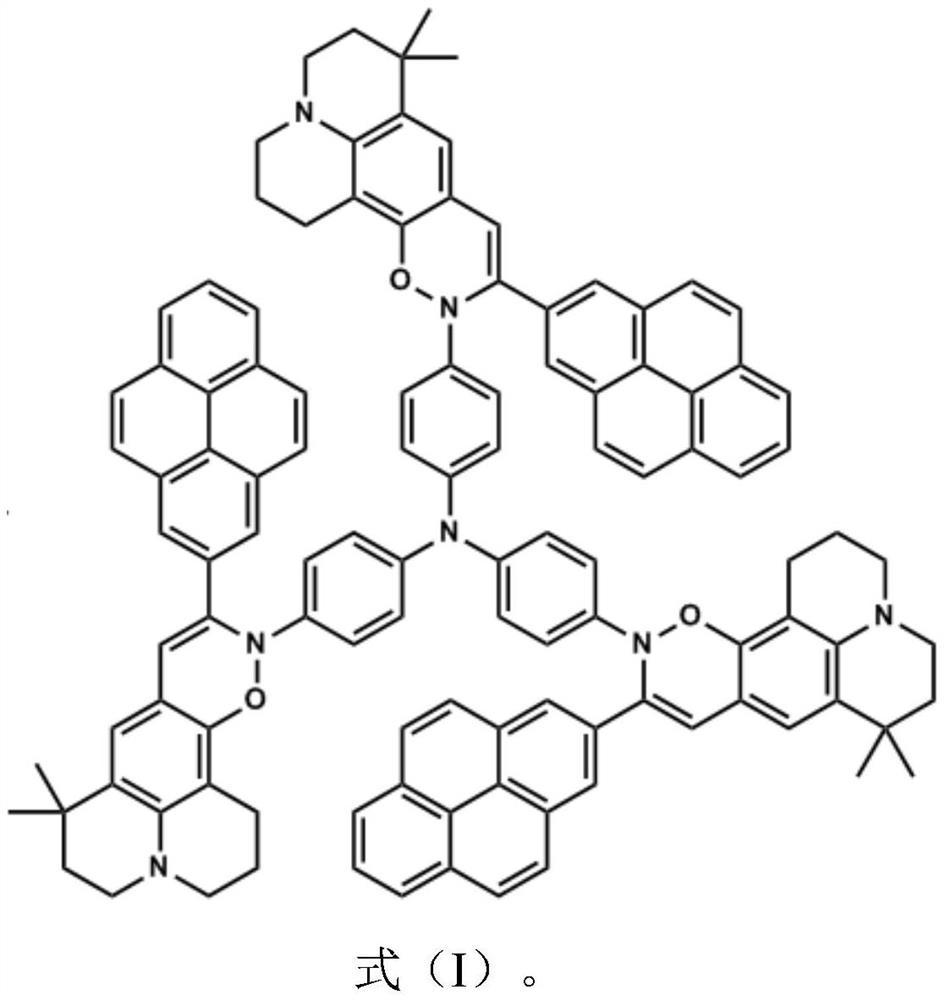
[0028] At room temperature, the compound (2.69g, 10mmol) shown in the formula (IV) was added in a 100ml single-port reaction flask, and then an appropriate amount of solvent THF was added until the compound shown in the formula (IV) was completely dissolved (about 80ml), and the liquid bromine ( 2ml, 40mmol) was dissolved in 3ml of THF solution, added dropwise to the reaction system, and reacted in the dark for 8 hours. Add water to stop the reaction, extract with DCM, wash several times with a large amount of deionized water, collect the organic liquid, and concentrate. The crude product is separated and purified by column chromatography to obtain a compound (2.089g, yield 60%) shown in formula (III), and its chemical reaction equation is:
[0029]
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com