Compound, thermal activation delayed fluorescence material and application thereof
A technology of thermally activated delayed and fluorescent materials, applied in the field of organic devices, can solve problems such as performance needs to be improved, and achieve the effects of improving luminous efficiency and working life, broadening the luminescent layer, and increasing the strength of the vibrator.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
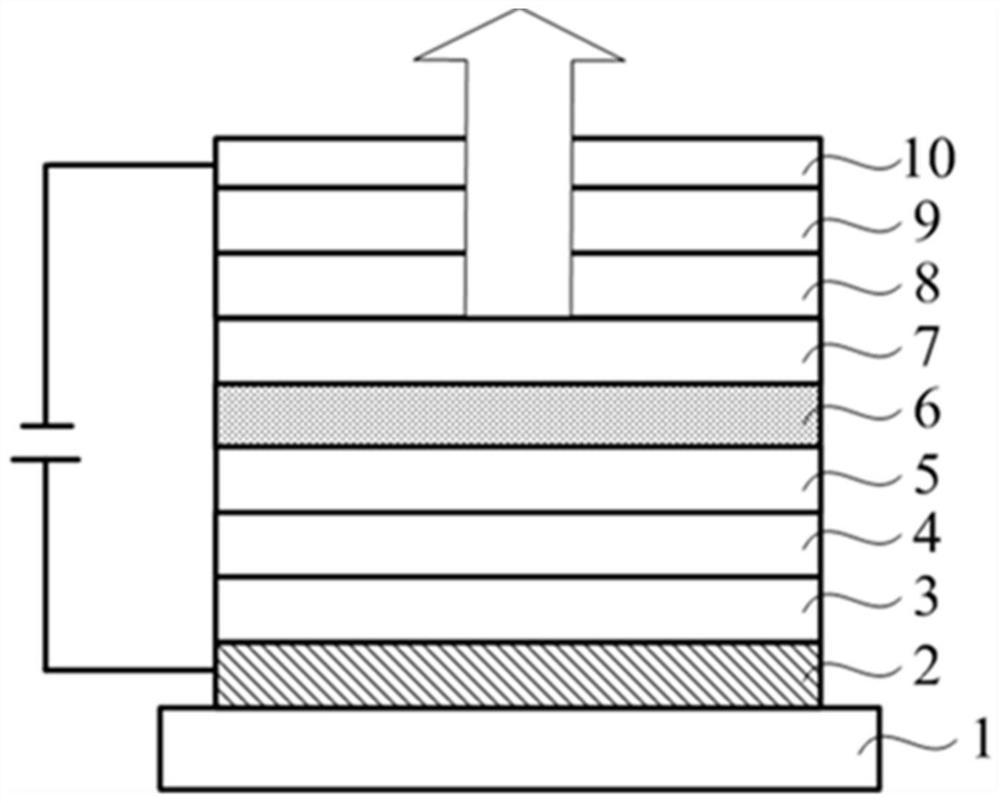
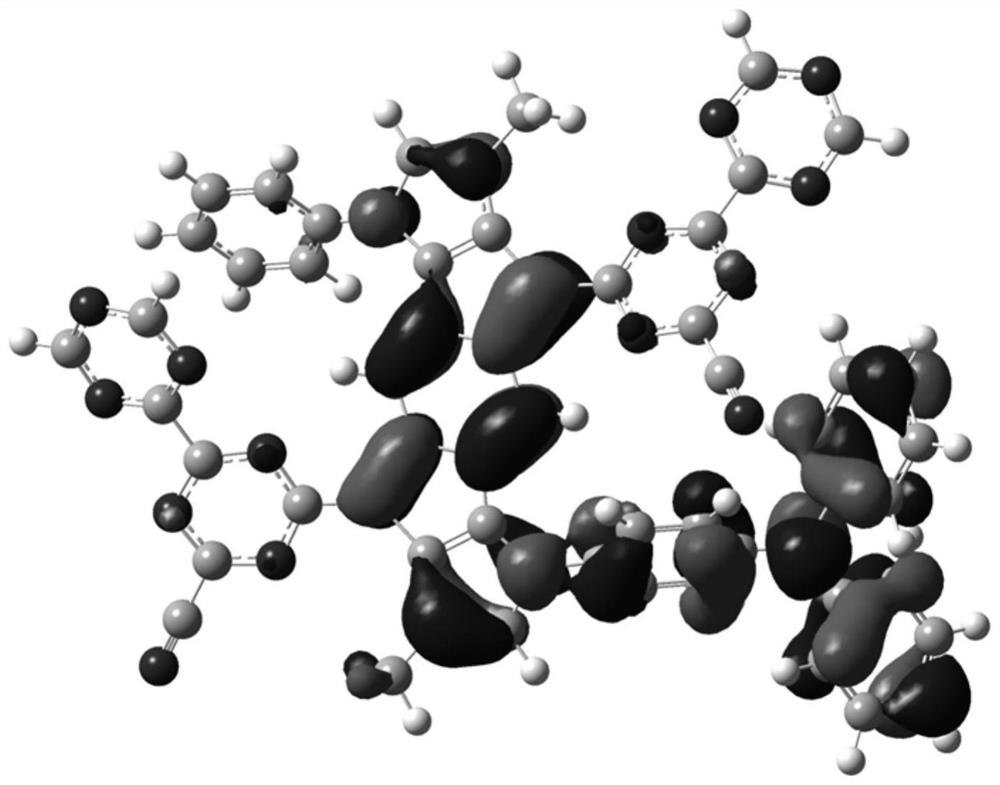
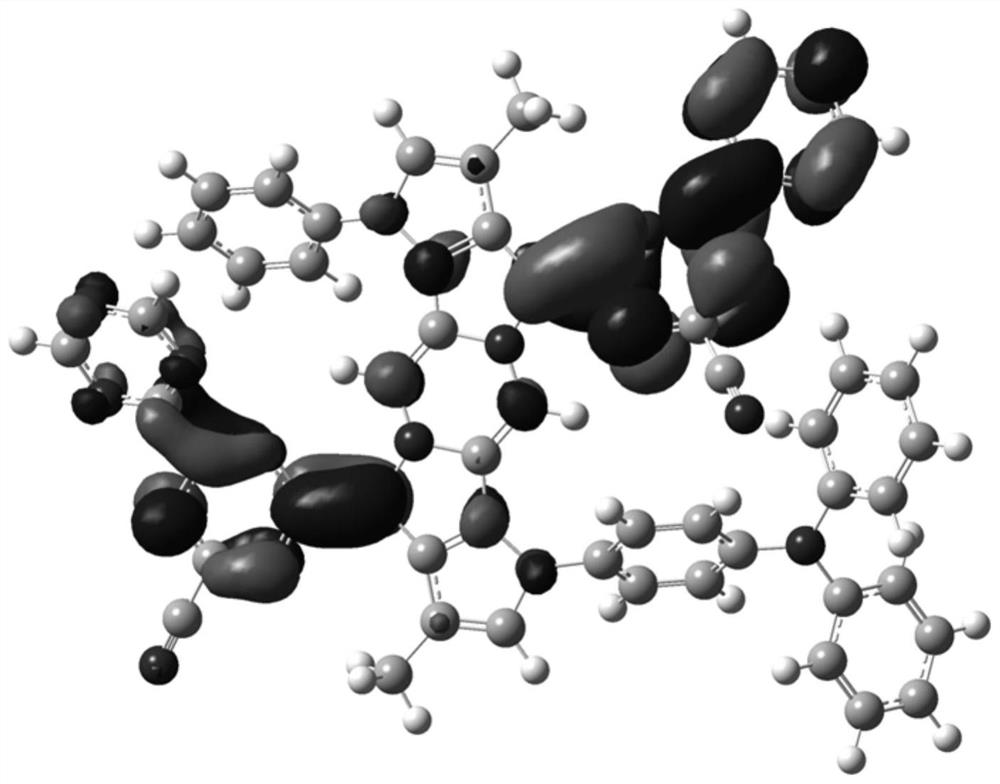
Image
Examples
preparation example 1
[0090] Synthesis of compound M3:
[0091]
[0092] (1) Add 5.60g (20mmol) of Compound A, 7.95g (20mmol) of Compound B, 8.94g (20mmol) of Compound C, 150mL of toluene, 13.81g (30mmol) of cesium carbonate, 0.23 g (0.2 mmol) of tetrakis(triphenylphosphine)palladium was reacted at 120° C. for 24 hours under a nitrogen atmosphere. Cool to room temperature, pour the reaction solution into 200mL ice water, extract three times with dichloromethane, combine the organic phases, spin into silica gel, and separate and purify by column chromatography (dichloromethane:n-hexane, v:v=1:1) to obtain Compound D.
[0093] 1 H NMR (400MHz, Chloroform) δ9.36(s,1H),9.08(s,1H),7.69(s,2H),7.58(s,1H),7.43(s,2H),7.36–7.29(m, 5H), 7.25(d, J=11.6Hz, 5H), 7.08(s, 2H), 7.00(s, 4H), 2.13(s, 6H).
[0094] 13 C NMR (100MHz, Chloroform) δ146.93(s), 140.83(s), 140.21(s), 134.42(s), 129.38–128.87(m), 127.54(s), 127.38–127.04(m), 125.65( s), 124.67(s), 122.99(s), 122.20(s), 121.16(s), 119.25(s), 95.28(s)...
preparation example 2
[0102] Synthesis of Compound M1
[0103]
[0104](1) Add 8.94g (20mmol) of Compound A, 3.98g (10mmol) of Compound B, 150mL of toluene, 13.81g (30mmol) of cesium carbonate, and tetrakis(triphenylphosphine) palladium in a 250mL three-necked flask. 0.23 g (0.2 mmol), and then reacted at 120° C. for 24 hours under a nitrogen atmosphere. Cool to room temperature, pour the reaction solution into 200mL ice water, extract three times with dichloromethane, combine the organic phases, spin into silica gel, and separate and purify by column chromatography (dichloromethane:n-hexane, v:v=1:1) to obtain Compound D.
[0105] 1 H NMR (400MHz, Chloroform) δ7.50–7.44(m,6H),7.32–7.24(m,8H),7.22–7.17(m,6H),7.14–7.07(m,10H),6.67(s,2H ),2.29(s,6H).
[0106] 13 C NMR (100MHz, Chloroform) δ146.34, 144.20, 137.71, 134.55, 134.13, 133.53, 130.17, 129.22, 127.64, 126.89, 125.87, 123.63, 122.29, 96.94, 14.03.
[0107] (2) In a 250ml three-neck flask, add 10.43g (10mmol) of substrate D (10mmol) a...
preparation example 3
[0114] Synthesis of compound M2
[0115]
[0116] (1) Add 9.22g (20mmol) of Compound A, 3.98g (10mmol) of Compound B, 150mL of toluene, 13.81g (30mmol) of cesium carbonate, and tetrakis(triphenylphosphine) palladium in a 250mL three-necked flask. 0.23 g (0.2 mmol), and then reacted at 120° C. for 24 hours under a nitrogen atmosphere. Cool to room temperature, pour the reaction solution into 200mL ice water, extract three times with dichloromethane, combine the organic phases, spin into silica gel, and separate and purify by column chromatography (dichloromethane:n-hexane, v:v=1:1) to obtain Compound D.
[0117] 1 H NMR (400MHz, Chloroform) δ7.52–7.46(m,4H),7.34–7.28(m,4H),7.15(dd,J=7.3,1.8Hz,6H),7.11(td,J=7.2,1.5 Hz,6H),7.07(td,J=7.3,1.8Hz,2H),6.78(dd,J=7.4,1.6Hz,4H),6.67(s,2H),2.29(s,6H).
[0118] 13 C NMR (100MHz, Chloroform) δ146.45, 139.72, 137.77, 134.55, 134.13, 133.40, 133.34, 130.17, 126.28, 126.22, 125.77, 123.92, 122.95, 120.19, 116.50, 946.034,
[0119] (2)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com