Organic electroluminescent compound and organic electroluminescent device
An electroluminescence and compound technology, applied in the field of organic electroluminescence materials, can solve the problems of poor stability of phosphorescent devices, unfavorable large-scale production, and high production costs, and achieves improved exciton utilization, skeleton stability, and reduced overlap. degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
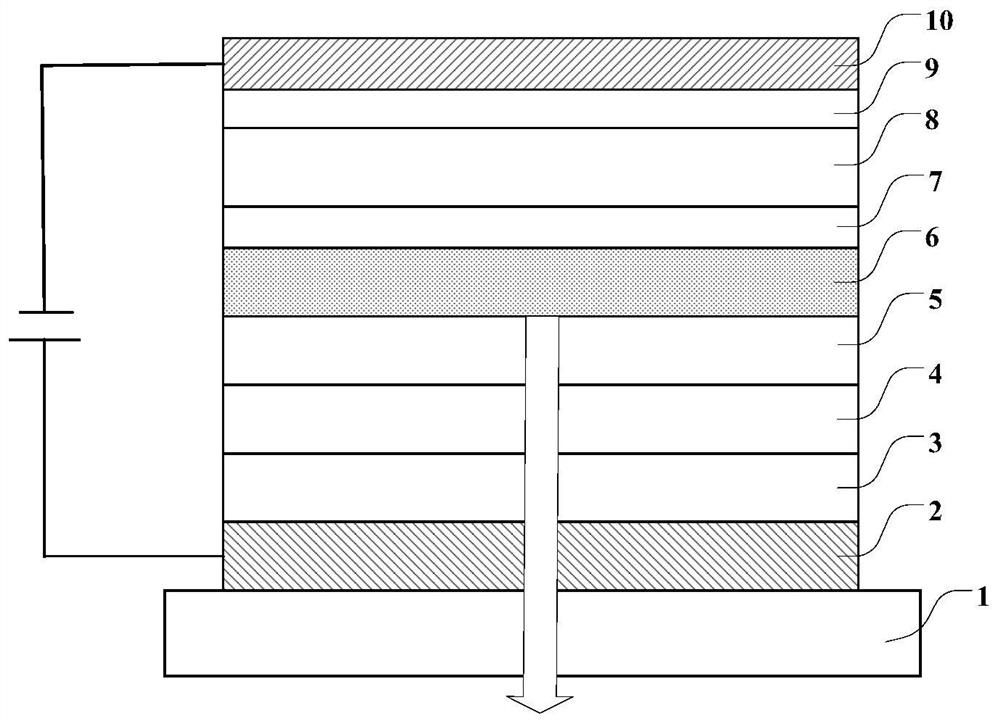
Image
Examples
preparation example Construction
[0079] The present invention also provides a method for preparing the above-mentioned organic electroluminescent compound, comprising: reacting the compound represented by the formula (II) and the compound represented by the formula (III) under the action of a Lewis acid catalyst to obtain the formula ( I) the compound shown; The Lewis acid catalyst is preferably aluminum trichloride.
[0080]
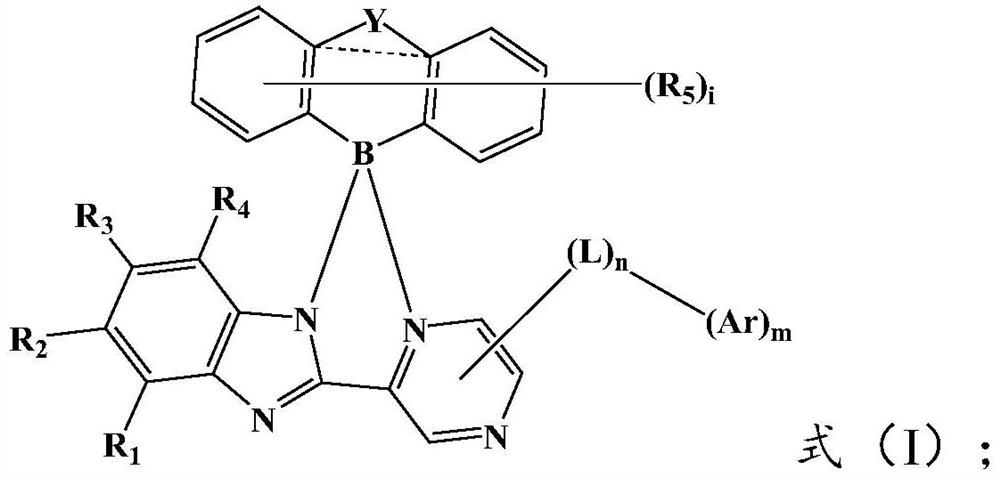
[0081] Wherein, the X is a halogen, preferably Cl or Br; the R 1 ~R 5 , Y, L and Ar are the same as above, and will not be repeated here.
[0082] The compound shown in the formula (II) is preferably prepared according to the following method: the compound shown in the formula (IV) and BX 3 React under the effect of acid-binding agent, obtain the compound shown in formula (II); Described acid-binding agent is preferably DIEA; Described reaction is preferably carried out in organic solvent; Described organic solvent is preferably dichloromethane; Said The reaction temperature is p...
Embodiment 1
[0109] Embodiment 1: preparation compound V21
[0110]
[0111] Under nitrogen atmosphere, add reactant 1 o-phenylenediamine (10mmol) and reactant 2 5-bromopyrazine-2-carbaldehyde (10mmol) into 100mL ethanol solvent, stir and dissolve, then add NH 4 Cl (25mmol) reactant, the mixture was stirred at 80°C for about 4h, and then monitored by spot plate. After the reaction was completed, the reaction solution was cooled to room temperature, and then poured into ice water, and a solid compound was precipitated. The reaction solution was suction filtered, washed twice with water, and dried; recrystallized with ethanol to obtain Intermediate A (yield 80%).
[0112] MALDI-TOF: m / z: Calculated: C11H7BrN4: 273.99, Found: 274.20.
[0113]
[0114] Under a nitrogen atmosphere, add 100mL of dioxane solvent into a 250mL reaction bottle, and then add K 2 CO 3 (5mmol)aq, intermediate A (2mmol), reactant 3 (2.4mmol), and Pd(PPh 3 ) 4 (0.10 mmol), heated to 100°C, and reacted overnigh...
Embodiment 2
[0122] Embodiment 2: preparation compound O21
[0123]
[0124] The difference between the preparation method of this compound O21 and Preparation Example 1 is that the raw material 4 in the step (4) of Preparation Example 1 is replaced with an equimolar amount of raw material 5, and other raw materials, reaction steps and reaction conditions are the same as in Example 1. , and finally obtained compound O21 (yield 69%).
[0125] MALDI-TOF: m / z: Calculated: C41H26BN5O: 615.22, Found: 615.41.
[0126] Compound elemental analysis results: calculated value: C41H26BN5O (%): C 80.01, H4.26, N 11.38; test value: C 80.00, H4.27, N 11.40.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com