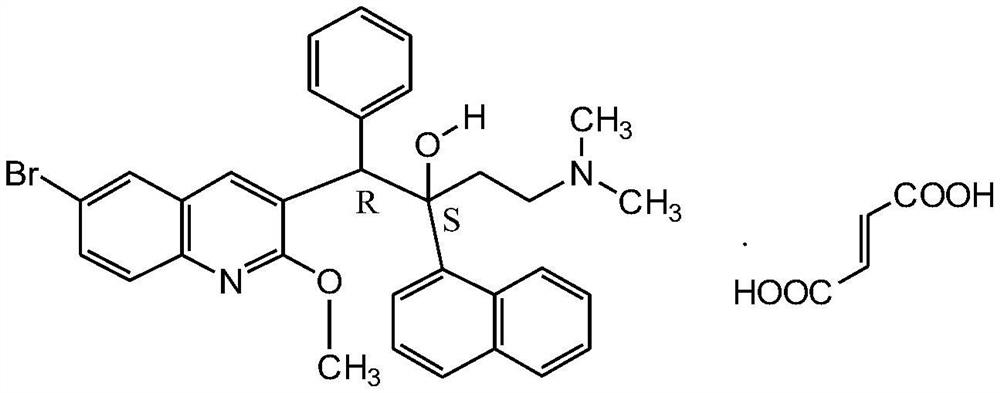
Combination in treatment of nontuberculous mycobacterial diseases
A mycobacterial, non-tuberculosis technology, used in medical preparations containing active ingredients, antibacterial drugs, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
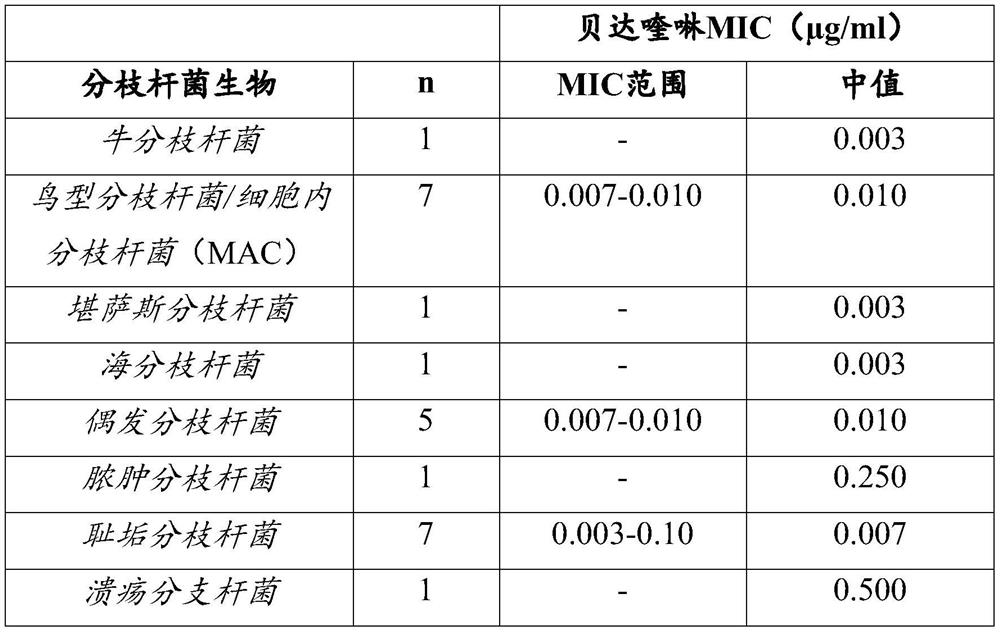
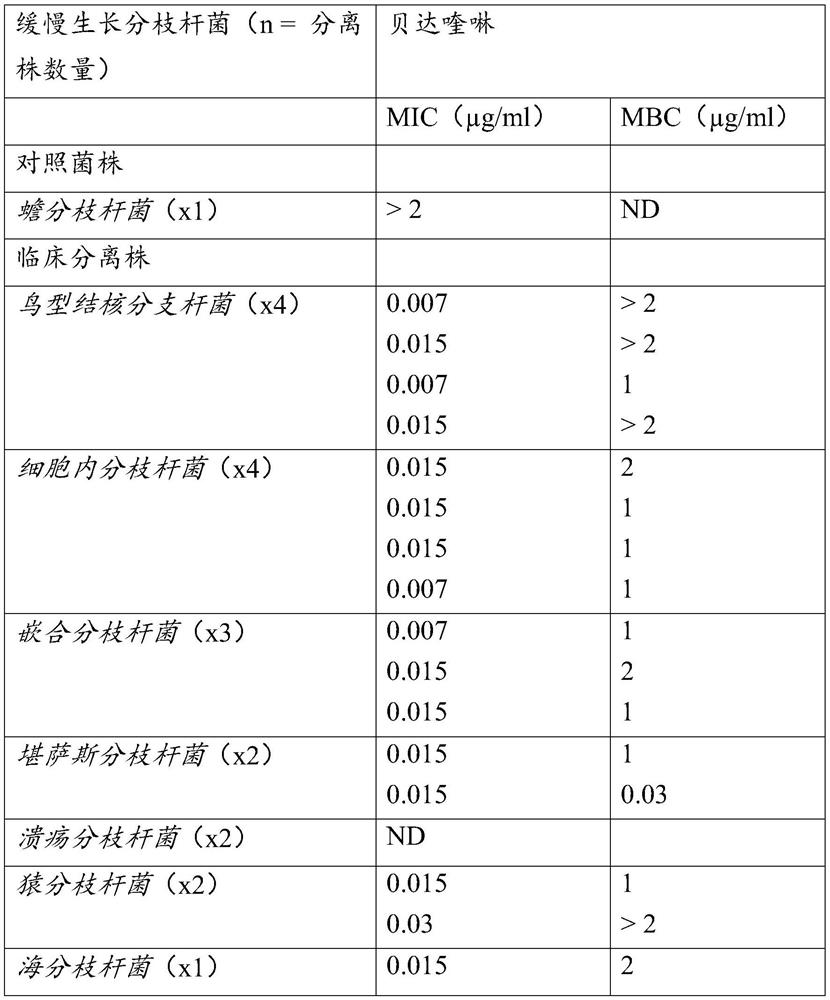
[0062] Reference example 1. the in vitro activity of bedaquiline
[0063] Bedaquiline is unique in its specificity for mycobacteria, including important atypical species in humans such as M. avium, M. kansasii, and the rapidly growing M. fortuitum and M. abscessus spectrum. M. avium, M. kansasii, and M. abscessus may cause NTM disease.
[0064] The minimum inhibitory concentration (MIC) of bedaquiline against Mycobacterium tuberculosis ranged from ≤0.008 μg / ml to 0.12 μg / ml, regardless of the drug-resistant subtype. Bedaquiline MIC is usually <0.1 μg / ml against other mycobacterial species (including species that are naturally resistant to many other anti-TB agents and are involved in opportunistic infections such as M. avium, M. abscessus) . Mycobacterium fortuitum and Mycobacterium marinum. One isolate each of M. abscessus (0.25 μg / ml) and M. ulcerans (0.50 μg / ml) was found to have a higher MIC compared to M. tuberculosis (see table below). The activity of bedaquiline ...
example 2
[0083] Example 2: In vivo testing
[0084] Purpose
[0085] the main purpose
[0086] The primary objective of this study was to evaluate the effect of bedaquiline amplified cyclic lactone (clarithromycin) and ethambutol (bedaquiline / clarithromycin / ethambutol) in combination with rifamycin amplified cyclic lactone Ester (clarithromycin) versus ethambutol (rifamycin / clarithromycin / ethambutol) in the treatment of NTM-PD in adult patients with refractory NTM-PD due to MAC effect.
[0087] secondary purpose
[0088] Secondary objectives are in adult patients with refractory NTM-PD due to MAC:
[0089] · Assess changes in quantitative sputum colony forming unit (CFU) counts during the 3-month and 6-month treatment periods with:
[0090] Bedaquiline / clarithromycin / ethambutol compared to rifamycin / clarithromycin / ethambutol.
[0091] ·Assess negative sputum culture at 1, 2, 3, 4, 5 months during treatment and at the end of 3-month follow-up after 12 months of treatment.
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com